Acrylonitrile butadiene styrene (ABS) and polycarbonate (PC) filaments three-dimensional (3-D) printer emissions-induced cell toxicity
- PMID: 31562913
- PMCID: PMC7490755
- DOI: 10.1016/j.toxlet.2019.09.013
Acrylonitrile butadiene styrene (ABS) and polycarbonate (PC) filaments three-dimensional (3-D) printer emissions-induced cell toxicity
Abstract
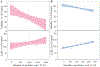
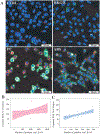
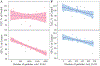
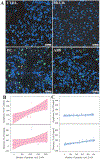
During extrusion of some polymers, fused filament fabrication (FFF) 3-D printers emit billions of particles per minute and numerous organic compounds. The scope of this study was to evaluate FFF 3-D printer emission-induced toxicity in human small airway epithelial cells (SAEC). Emissions were generated from a commercially available 3-D printer inside a chamber, while operating for 1.5 h with acrylonitrile butadiene styrene (ABS) or polycarbonate (PC) filaments, and collected in cell culture medium. Characterization of the culture medium revealed that repeat print runs with an identical filament yield various amounts of particles and organic compounds. Mean particle sizes in cell culture medium were 201 ± 18 nm and 202 ± 8 nm for PC and ABS, respectively. At 24 h post-exposure, both PC and ABS emissions induced a dose dependent significant cytotoxicity, oxidative stress, apoptosis, necrosis, and production of pro-inflammatory cytokines and chemokines in SAEC. Though the emissions may not completely represent all possible exposure scenarios, this study indicate that the FFF could induce toxicological effects. Further studies are needed to quantify the detected chemicals in the emissions and their corresponding toxicological effects.
Keywords: Emerging technologies; In vitro toxicity; Inflammatory response; Printer emitted nanoparticles.
Copyright © 2019 Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest
The authors declare no competing financial interest.
Figures
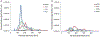
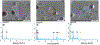

References
-
- ASTM-D6670, 2013. ASTM D6670: Standard Practice for Full-scale Chamber Determination of Volatile Organic Emissions from Indoor Materials/Products. ASTM International, West Conshohocken, PA.
-
- Azimi P, Zhao D, Pouzet C, Crain NE, Stephens B, 2016. Emissions of ultrafine particles and volatile organic compounds from commercially available desktop three-dimensional printers with multiple filaments. Environ. Sci. Technol 50, 1260–1268. - PubMed
-
- Barnes PJ, 2009. The cytokine network in chronic obstructive pulmonary disease. Am. J. Respir. Cell Mol. Biol 41, 631–638. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

