Input-Specific Metaplasticity in the Visual Cortex Requires Homer1a-Mediated mGluR5 Signaling
- PMID: 31563294
- PMCID: PMC6872932
- DOI: 10.1016/j.neuron.2019.08.017
Input-Specific Metaplasticity in the Visual Cortex Requires Homer1a-Mediated mGluR5 Signaling
Abstract
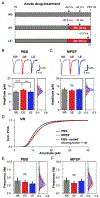
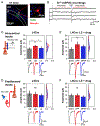
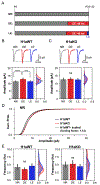
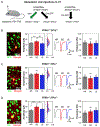
Effective sensory processing depends on sensory experience-dependent metaplasticity, which allows homeostatic maintenance of neural network activity and preserves feature selectivity. Following a strong increase in sensory drive, plasticity mechanisms that decrease the strength of excitatory synapses are preferentially engaged to maintain stability in neural networks. Such adaptation has been demonstrated in various model systems, including mouse primary visual cortex (V1), where excitatory synapses on layer 2/3 (L2/3) neurons undergo rapid reduction in strength when visually deprived mice are reexposed to light. Here, we report that this form of plasticity is specific to intracortical inputs to V1 L2/3 neurons and depends on the activity of NMDA receptors (NMDARs) and group I metabotropic glutamate receptor 5 (mGluR5). Furthermore, we found that expression of the immediate early gene (IEG) Homer1a (H1a) and its subsequent interaction with mGluR5s are necessary for this input-specific metaplasticity.
Keywords: H1a; NMDA receptor; mGluR5; metabotropic glutamate receptor; metaplasticity; visual cortex.
Copyright © 2019 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests
PFW is a co-founder and serving as a Chief Scientific Officer of CogNext.
Figures


References
-
- Abbott LF, and Nelson SB (2000). Synaptic plasticity: taming the beast. Nature neuroscience 3 Suppl, 1178–1183. - PubMed
-
- Abraham WC (2008). Metaplasticity: tuning synapses and networks for plasticity. Nature reviews Neuroscience 9, 387. - PubMed
-
- Ango F, Prézeau L, Muller T, Tu JC, Xiao B, Worley PF, Pin JP, Bockaert J, and Fagni L (2001). Agonist-independent activation of metabotropic glutamate receptors by the intracellular protein Homer. Nature 411, 35082096. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

