A head-to-head comparison of the efficacy and safety of ixekizumab and adalimumab in biological-naïve patients with active psoriatic arthritis: 24-week results of a randomised, open-label, blinded-assessor trial
- PMID: 31563894
- PMCID: PMC6937408
- DOI: 10.1136/annrheumdis-2019-215386
A head-to-head comparison of the efficacy and safety of ixekizumab and adalimumab in biological-naïve patients with active psoriatic arthritis: 24-week results of a randomised, open-label, blinded-assessor trial
Abstract
Objectives: To compare efficacy and safety of ixekizumab (IXE) to adalimumab (ADA) in biological disease-modifying antirheumatic drug-naïve patients with both active psoriatic arthritis (PsA) and skin disease and inadequate response to conventional synthetic disease-modifying antirheumatic drug (csDMARDs).
Methods: Patients with active PsA were randomised (1:1) to approved dosing of IXE or ADA in an open-label, head-to-head, blinded assessor clinical trial. The primary objective was to evaluate whether IXE was superior to ADA at week 24 for simultaneous achievement of a ≥50% improvement from baseline in the American College of Rheumatology criteria (ACR50) and a 100% improvement from baseline in the Psoriasis Area and Severity Index (PASI100). Major secondary objectives, also at week 24, were to evaluate whether IXE was: (1) non-inferior to ADA for achievement of ACR50 and (2) superior to ADA for PASI100 response. Additional PsA, skin, treat-to-target and quality-of-life outcome measures were assessed at week 24.
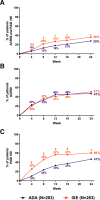
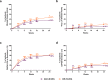
Results: The primary efficacy endpoint was met (IXE: 36%, ADA: 28%; p=0.036). IXE was non-inferior for ACR50 response (IXE: 51%, ADA: 47%; treatment difference: 3.9%) and superior for PASI100 response (IXE: 60%, ADA: 47%; p=0.001). IXE had greater response versus ADA in additional PsA, skin, nail, treat-to-target and quality-of-life outcomes. Serious adverse events were reported in 8.5% (ADA) and 3.5% (IXE) of patients.
Conclusions: IXE was superior to ADA in achievement of simultaneous improvement of joint and skin disease (ACR50 and PASI100) in patients with PsA and inadequate response to csDMARDs. Safety and tolerability for both biologicals were aligned with established safety profiles.
Keywords: adalimumab; clinical trial; head-to-head; ixekizumab; psoriatic arthritis.
© Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: PJM reports research grants and personal fees from AbbVie, Amgen, Bristol Myers Squibb, Celgene, Janssen and Lilly; and personal fees from Boehringer Ingelheim, Galapagos, Genentech and Gilead. JSS reports research grants from AbbVie, Astra-Zeneca, Janssen, Lilly, MSD, Novartis, Pfizer and Roche; and personal fees from AbbVie, Amgen, Astra-Zeneca, Astro, BMS, Celgene, Celltrion, Chugai, Gilead, ILTOO, Janssen, Lilly, MSD, Novartis-Sandoz, Pfizer, Roche, Samsung, Sanofi and UCB. FB reports research grants from Pfizer, Janssen, Chugai, Celgene and Roche; personal fees from Pfizer, AbbVie, Sanofi, Lilly, Novartis, Genzyme, Boehringer, Janssen, MSD, Celgene, Roche and Chugai; and investigator fees from Lilly. PN reports research grants and personal fees from AbbVie, BMS, Janssen, Lilly, MSD, Novartis, Pfizer, Celgene, Gilead, Sanofi, UCB and Roche. HT reports research grants and non-financial support from Lilly. MG reports personal fees from AbbVie, Actelion Pharmaceuticals, Akros Pharma Inc, AMGEN Inc, Arcutis Pharmaceuticals Inc, Boehringer Engelheim International GmbH, Bristol-Myers Squibb Company, Celgene corporation, Dermira Inc, Eli Lilly and Company, Galderma, GlaxoSmithKline, Glenmark, Jannsen Inc, LEO Pharma, MedImmune, Merck and Co, Novartis Pharmaceuticals, Pfizer Inc, Regeneron Pharmaceuticals Inc, Roche Laboratories, Sanofi Genzyme, UCB and Valeant Pharmaceuticals Inc. PE has undertaken clinical trials and provided expert advice to Pfizer, MSD, Abbvie, BMS, UCB, Roche, Novartis, Samsung, Sandoz and Lilly; has received consultant fees from BMS, AbbVie, Pfizer, MSD, Novartis, Roche and UCB; and has received research grants paid to his employer from AbbVie, BMS, Pfizer, MSD and Roche. PSH reports research grants, personal fees and non-financial support from AbbVie; research grants from Amgen, Janssen, Pfizer and UCB; and personal fees from Lilly and Galapagos. SLL, LL, EK, HL-S and SP are employees of, and own stock in, Eli Lilly and Company.
Figures
References
-
- Kavanaugh A, Gottlieb A, Morita A, et al. . The contribution of joint and skin improvements to the health-related quality of life of patients with psoriatic arthritis: a post hoc analysis of two randomised controlled studies. Ann Rheum Dis 2019;78:1215–9. 10.1136/annrheumdis-2018-215003 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

