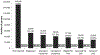Three Years of Progress Toward Achieving Hepatitis C Elimination in the Country of Georgia, April 2015-March 2018
- PMID: 31563938
- PMCID: PMC7484896
- DOI: 10.1093/cid/ciz956
Three Years of Progress Toward Achieving Hepatitis C Elimination in the Country of Georgia, April 2015-March 2018
Abstract
Background: In April 2015, in collaboration with the US Centers for Disease Control and Prevention and Gilead Sciences, the country of Georgia embarked on the world's first hepatitis C elimination program. We aimed to assess progress toward elimination targets 3 years after the start of the elimination program.
Methods: We constructed a hepatitis C virus (HCV) care cascade for adults in Georgia, based on the estimated 150 000 persons aged ≥18 years with active HCV infection. All patients who were screened or entered the treatment program during April 2015-March 2018 were included in the analysis. Data on the number of persons screened for HCV were extracted from the national HCV screening database. For the treatment component, we utilized data from the Georgia National HCV treatment program database. Available treatment options included sofosbuvir and ledipasvir/sofosbuvir-based regimens.
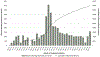
Results: Since April 2015, a cumulative 974 817 adults were screened for HCV antibodies; 86 624 persons tested positive, of whom 61 925 underwent HCV confirmatory testing. Among the estimated 150 000 adults living with chronic hepatitis C in Georgia, 52 856 (35.1%) were diagnosed, 45 334 (30.2%) initiated treatment with direct-acting antivirals, and 29 090 (19.4%) achieved a sustained virologic response (SVR). Overall, 37 256 persons were eligible for SVR assessment; of these, only 29 620 (79.5%) returned for evaluation. The SVR rate was 98.2% (29 090/29 620) in the per-protocol analysis and 78.1% (29 090/37 256) in the intent-to-treat analysis.
Conclusions: Georgia has made substantial progress in the path toward eliminating hepatitis C. Scaling up of testing and diagnosis, along with effective linkage to treatment services, is needed to achieve the goal of elimination.
Keywords: Georgia; HCV; cascade; elimination.
© The Author(s) 2019. Published by Oxford University Press for the Infectious Diseases Society of America. All rights reserved. For permissions, e-mail: journals.permissions@oup.com.
Conflict of interest statement
Figures
Comment in
-
Hepatitis C Virus Elimination Requires More Than Good Drugs.Clin Infect Dis. 2020 Aug 22;71(5):1269-1270. doi: 10.1093/cid/ciz963. Clin Infect Dis. 2020. PMID: 31563937 Free PMC article. No abstract available.
References
-
- Pawlotsky JM, Feld JJ, Zeuzem S, Hoofnagle JH. From non-A, non-B hepatitis to hepatitis C virus cure. J Hepatol 2015; 62:S87–99. - PubMed
-
- World Health Organization. Global health sector strategy on viral hepatitis 2016–2021. Geneva, Switzerland: WHO, 2016.
-
- Bulterys M Global progress towards hepatitis elimination—an update In: The Intentional Liver Congress 2019. International Liver Congress, Vienna, Austria, 10–14 April 2019.
-
- CDA Foundation. Polaris observatory. Available at: http://cdafound.org/polaris/. Accessed 20 April 2019.
-
- Cooke GS, Andrieux-Meyer I, Applegate TL, et al. Lancet Gastroenterology and Hepatology Commissioners. Accelerating the elimination of viral hepatitis: a Lancet Gastroenterology and Hepatology Commission. Lancet Gastroenterol Hepatol 2019; 4:135–84. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous