TSPO deficiency induces mitochondrial dysfunction, leading to hypoxia, angiogenesis, and a growth-promoting metabolic shift toward glycolysis in glioblastoma
- PMID: 31563962
- PMCID: PMC7442372
- DOI: 10.1093/neuonc/noz183
TSPO deficiency induces mitochondrial dysfunction, leading to hypoxia, angiogenesis, and a growth-promoting metabolic shift toward glycolysis in glioblastoma
Erratum in
-
Correction to: TSPO deficiency induces mitochondrial dysfunction, leading to hypoxia, angiogenesis, and a growth-promoting metabolic shift toward glycolysis in glioblastoma.Neuro Oncol. 2024 Apr 5;26(4):777-778. doi: 10.1093/neuonc/noad251. Neuro Oncol. 2024. PMID: 38175620 Free PMC article. No abstract available.
Abstract
Background: The ligands of mitochondrial translocator protein (TSPO) have been widely used as diagnostic biomarkers for glioma. However, the true biological actions of TSPO in vivo and its role in glioma tumorigenesis remain elusive.
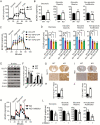
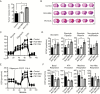
Methods: TSPO knockout xenograft and spontaneous mouse glioma models were employed to assess the roles of TSPO in the pathogenesis of glioma. A Seahorse Extracellular Flux Analyzer was used to evaluate mitochondrial oxidative phosphorylation and glycolysis in TSPO knockout and wild-type glioma cells.
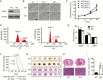
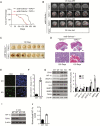
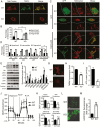
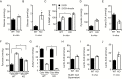
Results: TSPO deficiency promoted glioma cell proliferation in vitro in mouse GL261 cells and patient-derived stem cell-like GBM1B cells. TSPO knockout increased glioma growth and angiogenesis in intracranial xenografts and a mouse spontaneous glioma model. Loss of TSPO resulted in a greater number of fragmented mitochondria, increased glucose uptake and lactic acid conversion, decreased oxidative phosphorylation, and increased glycolysis.
Conclusion: TSPO serves as a key regulator of glioma growth and malignancy by controlling the metabolic balance between mitochondrial oxidative phosphorylation and glycolysis.1. TSPO deficiency promotes glioma growth and angiogenesis.2. TSPO regulates the balance between mitochondrial oxidative phosphorylation and glycolysis.
Keywords: TSPO; angiogenesis; glioma; glycolysis; mitochondrial oxidative phosphorylation.
© The Author(s) 2019. Published by Oxford University Press on behalf of the Society for Neuro-Oncology. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures
References
-
- Lacapère JJ, Papadopoulos V. Peripheral-type benzodiazepine receptor: structure and function of a cholesterol-binding protein in steroid and bile acid biosynthesis. Steroids. 2003;68(7–8):569–585. - PubMed
-
- Kelly-Hershkovitz E, Weizman R, Spanier I, et al. . Effects of peripheral-type benzodiazepine receptor antisense knockout on MA-10 Leydig cell proliferation and steroidogenesis. J Biol Chem. 1998;273(10):5478–5483. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

