High-gradient diffusion MRI reveals distinct estimates of axon diameter index within different white matter tracts in the in vivo human brain
- PMID: 31563995
- PMCID: PMC7101261
- DOI: 10.1007/s00429-019-01961-2
High-gradient diffusion MRI reveals distinct estimates of axon diameter index within different white matter tracts in the in vivo human brain
Abstract
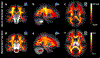
Axon diameter and density are important microstructural metrics that offer valuable insight into the structural organization of white matter throughout the human brain. We report the systematic acquisition and analysis of a comprehensive diffusion MRI data set acquired with 300 mT/m maximum gradient strength in a cohort of 20 healthy human subjects that yields distinct and consistent patterns of axon diameter index in white matter tracts of arbitrary orientation. We use a straightforward, previously validated approach to estimating indices of axon diameter and volume fraction that involves interpolating the diffusion signal perpendicular to the principal fiber orientation and fitting a three-compartment model of intra-axonal, extra-axonal and free water diffusion. The resultant maps confirm the presence of larger diameter indices in the body of corpus callosum compared to the genu and splenium, as previously reported, and show larger axon diameter index in the corticospinal tracts compared to adjacent white matter tracts such as the cingulum. An anterior-to-posterior gradient in axon diameter index is also observed, with smaller diameter indices in the frontal lobes and larger diameter indices in the parieto-occipital white matter. These observations are consistent with known trends from prior histologic studies in humans and non-human primates. Rather than serving as fully quantitative measures of axon diameter and density, our results may be considered as axon diameter- and volume fraction-weighted images that appear to be modulated by the underlying microstructure and may capture broad trends in axonal size and packing density, acknowledging that the precise origin of such modulation requires further investigation that will be facilitated by the availability of high gradient strengths for in vivo human imaging.
Keywords: Axon diameter index; Diffusion; Human brain; In vivo; MRI.
Figures




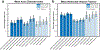
Similar articles
-
Age-related alterations in axonal microstructure in the corpus callosum measured by high-gradient diffusion MRI.Neuroimage. 2019 May 1;191:325-336. doi: 10.1016/j.neuroimage.2019.02.036. Epub 2019 Feb 18. Neuroimage. 2019. PMID: 30790671 Free PMC article.
-
The impact of gradient strength on in vivo diffusion MRI estimates of axon diameter.Neuroimage. 2015 Feb 1;106:464-72. doi: 10.1016/j.neuroimage.2014.12.008. Epub 2014 Dec 9. Neuroimage. 2015. PMID: 25498429 Free PMC article.
-
Scan-rescan repeatability of axonal imaging metrics using high-gradient diffusion MRI and statistical implications for study design.Neuroimage. 2021 Oct 15;240:118323. doi: 10.1016/j.neuroimage.2021.118323. Epub 2021 Jul 1. Neuroimage. 2021. PMID: 34216774 Free PMC article.
-
Connectome 2.0: Developing the next-generation ultra-high gradient strength human MRI scanner for bridging studies of the micro-, meso- and macro-connectome.Neuroimage. 2021 Nov;243:118530. doi: 10.1016/j.neuroimage.2021.118530. Epub 2021 Aug 28. Neuroimage. 2021. PMID: 34464739 Free PMC article. Review.
-
Towards in vivo g-ratio mapping using MRI: Unifying myelin and diffusion imaging.J Neurosci Methods. 2021 Jan 15;348:108990. doi: 10.1016/j.jneumeth.2020.108990. Epub 2020 Oct 28. J Neurosci Methods. 2021. PMID: 33129894 Free PMC article. Review.
Cited by
-
Age-related alterations in human cortical microstructure across the lifespan: Insights from high-gradient diffusion MRI.Aging Cell. 2024 Nov;23(11):e14267. doi: 10.1111/acel.14267. Epub 2024 Aug 8. Aging Cell. 2024. PMID: 39118344 Free PMC article.
-
Oscillating gradient spin echo diffusion time effects implicate variations in neurite beading for the heterogeneous reduced diffusion in human acute ischemic stroke lesions.Magn Reson Med. 2025 Nov;94(5):2158-2172. doi: 10.1002/mrm.30618. Epub 2025 Jun 24. Magn Reson Med. 2025. PMID: 40554725 Free PMC article.
-
Resolution and b value dependent structural connectome in ex vivo mouse brain.Neuroimage. 2022 Jul 15;255:119199. doi: 10.1016/j.neuroimage.2022.119199. Epub 2022 Apr 10. Neuroimage. 2022. PMID: 35417754 Free PMC article.
-
Quantifying axonal features of human superficial white matter from three-dimensional multibeam serial electron microscopy data assisted by deep learning.Neuroimage. 2025 Jun;313:121212. doi: 10.1016/j.neuroimage.2025.121212. Epub 2025 Apr 11. Neuroimage. 2025. PMID: 40222502 Free PMC article.
-
Longitudinal changes in cortical cell body and neurite density in people with multiple sclerosis.Neuroscience. 2025 Aug 30;582:195-202. doi: 10.1016/j.neuroscience.2025.07.027. Epub 2025 Jul 19. Neuroscience. 2025. PMID: 40691883
References
-
- Aboitiz F, Rodriguez E, Olivares R, Zaidel E (1996) Age-related changes in fibre composition of the human corpus callosum: sex differences Neuroreport 7:1761–1764 - PubMed
-
- Aboitiz F, Scheibel AB, Fisher RS, Zaidel E (1992) Fiber composition of the human corpus callosum Brain research 598:143–153 - PubMed
-
- Alexander DC (2008) A general framework for experiment design in diffusion MRI and its application in measuring direct tissue-microstructure features Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine 60:439–448 10.1002/mrm.21646 - DOI - PubMed
MeSH terms
Grants and funding
- R01NS095985/NS/NINDS NIH HHS/United States
- K23 NS078044/NS/NINDS NIH HHS/United States
- U01MH093765/MH/NIMH NIH HHS/United States
- R00EB015445/EB/NIBIB NIH HHS/United States
- S10RR023043/NH/NIH HHS/United States
- R01 NS095985/NS/NINDS NIH HHS/United States
- S10 RR023043/RR/NCRR NIH HHS/United States
- U01 MH093765/MH/NIMH NIH HHS/United States
- R01 HL131635/HL/NHLBI NIH HHS/United States
- K23NS078044/NS/NINDS NIH HHS/United States
- R01EB006847/EB/NIBIB NIH HHS/United States
- R56 HL125590/HL/NHLBI NIH HHS/United States
- S10RR023401/NH/NIH HHS/United States
- K23NS096056/NS/NINDS NIH HHS/United States
- S10 RR019307/RR/NCRR NIH HHS/United States
- R00 EB015445/EB/NIBIB NIH HHS/United States
- U01 EB026996/EB/NIBIB NIH HHS/United States
- R01 EB006847/EB/NIBIB NIH HHS/United States
- U01EB026996/NH/NIH HHS/United States
- P41EB015896/RR/NCRR NIH HHS/United States
- K23 NS096056/NS/NINDS NIH HHS/United States
- S10RR019307/NH/NIH HHS/United States
- P41 EB015896/EB/NIBIB NIH HHS/United States
- S10 RR023401/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

