Efficacy and safety of dupilumab in Japanese adults with moderate-to-severe atopic dermatitis: a subanalysis of three clinical trials
- PMID: 31564057
- PMCID: PMC7384164
- DOI: 10.1111/bjd.18565
Efficacy and safety of dupilumab in Japanese adults with moderate-to-severe atopic dermatitis: a subanalysis of three clinical trials
Abstract
Background: Dupilumab, a human monoclonal antibody, blocks the shared receptor unit for interleukin-4 and interleukin-13. International phase II and III studies have evaluated the efficacy and safety of dupilumab in adults with moderate-to-severe atopic dermatitis (AD), but the effects of dupilumab in Japanese patients have not been reported.
Objectives: To evaluate the efficacy and safety of dupilumab in Japanese patients with moderate-to-severe AD.
Methods: We analysed the efficacy and safety of dupilumab in the Japanese cohorts of a 16-week, phase IIb dose-finding trial (AD-1021; NCT01859988); a 16-week, phase III, placebo-controlled monotherapy trial (LIBERTY AD SOLO 1; NCT02277743) and a 52-week, phase III, placebo-controlled study of dupilumab with topical corticosteroids (LIBERTY AD CHRONOS; NCT02260986).
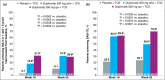
Results: Twenty-seven, 106 and 117 Japanese patients were enrolled in AD-1021, SOLO 1 and CHRONOS, respectively. Baseline disease severity was numerically higher in the Japanese cohort than in the overall study population. Generally, dupilumab significantly improved signs and symptoms of AD, including pruritus and patient quality of life, compared with placebo in the Japanese cohort, consistent with the overall study population. The combined safety profile of dupilumab in the Japanese cohort was similar to that in the total study populations; dupilumab was associated with an increased incidence of injection-site reactions and conjunctivitis compared with placebo. Dupilumab was associated with rapid reduction in thymus and activation-regulated chemokine and gradual IgE reductions.
Conclusions: Dupilumab alone or with topical corticosteroids improved signs and symptoms of AD, had an acceptable safety profile, and suppressed biomarkers of type 2 inflammation compared with placebo in Japanese adult patients with moderate-to-severe AD. What's already known about this topic? Differences in atopic dermatitis (AD) pathology have been reported between Asian and Western populations, in which distinct helper T-cell activation profiles have been observed. International clinical studies in adults with moderate-to-severe AD have evaluated the efficacy and safety of dupilumab, which blocks interleukin-4 and interleukin-13, key molecules in type 2 inflammation. The effects of dupilumab in Japanese patients specifically have not yet been reported. What does this study add? Dupilumab alone or with topical corticosteroids improved signs and symptoms of AD and had an acceptable safety profile compared with placebo in Japanese patients with moderate-to-severe AD. The effects were comparable with those observed in the overall study population. Reported immunological differences in AD pathology in Asian patients may be secondary to type 2 immune activation.
© 2019 The Authors. British Journal of Dermatology published by John Wiley & Sons Ltd on behalf of British Association of Dermatologists.
Figures







Similar articles
-
Dupilumab Provides Acceptable Safety and Sustained Efficacy for up to 4 Years in an Open-Label Study of Adults with Moderate-to-Severe Atopic Dermatitis.Am J Clin Dermatol. 2022 May;23(3):393-408. doi: 10.1007/s40257-022-00685-0. Epub 2022 May 3. Am J Clin Dermatol. 2022. PMID: 35503163 Free PMC article. Clinical Trial.
-
Long-term management of moderate-to-severe atopic dermatitis with lebrikizumab and concomitant topical corticosteroids: a 68-week randomized double-blind placebo-controlled phase III trial in Japan (ADhere-J).Br J Dermatol. 2025 Mar 18;192(4):597-610. doi: 10.1093/bjd/ljae394. Br J Dermatol. 2025. PMID: 39442013 Clinical Trial.
-
Conjunctivitis in Adults with Atopic Dermatitis Treated with Dupilumab: An Observational Study of Clinical Characteristics, Symptomatology, and Treatment.Adv Ther. 2025 Jul;42(7):3285-3305. doi: 10.1007/s12325-025-03209-4. Epub 2025 May 19. Adv Ther. 2025. PMID: 40388089 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Systemic treatments for eczema: a network meta-analysis.Cochrane Database Syst Rev. 2020 Sep 14;9(9):CD013206. doi: 10.1002/14651858.CD013206.pub2. Cochrane Database Syst Rev. 2020. PMID: 32927498 Free PMC article.
Cited by
-
Convergence of Neuroinflammation, Microbiota, and Parkinson's Disease: Therapeutic Insights and Prospects.Int J Mol Sci. 2024 Oct 29;25(21):11629. doi: 10.3390/ijms252111629. Int J Mol Sci. 2024. PMID: 39519181 Free PMC article. Review.
-
Response to Biologic Therapy in Skin of Colour Participants With Moderate-to-Severe Psoriasis and Atopic Dermatitis: A Systematic Review.J Cutan Med Surg. 2024 Sep-Oct;28(5):468-472. doi: 10.1177/12034754241260023. Epub 2024 Jun 7. J Cutan Med Surg. 2024. PMID: 38847375 Free PMC article.
-
Atopic Dermatitis Across Shades of Skin.Am J Clin Dermatol. 2023 Sep;24(5):731-751. doi: 10.1007/s40257-023-00797-1. Epub 2023 Jun 19. Am J Clin Dermatol. 2023. PMID: 37336869 Review.
-
A Literature Review of Real-World Effectiveness and Safety of Dupilumab for Atopic Dermatitis.JID Innov. 2021 Jul 30;1(3):100042. doi: 10.1016/j.xjidi.2021.100042. eCollection 2021 Sep. JID Innov. 2021. PMID: 34909737 Free PMC article. Review.
-
Barrier Factors of Adherence to Dupilumab Self-Injection for Severe Allergic Disease: A Non-Interventional Open-Label Study.Patient Prefer Adherence. 2023 Mar 27;17:861-872. doi: 10.2147/PPA.S389865. eCollection 2023. Patient Prefer Adherence. 2023. PMID: 37009430 Free PMC article.
References
-
- Saeki H, Nakahara T, Tanaka A et al Clinical practice guidelines for the management of atopic dermatitis 2016. J Dermatol 2016; 43:1117–45. - PubMed
-
- Katayama I, Kohno Y, Akiyama K et al Japanese guideline for atopic dermatitis 2014. Allergol Int 2014; 63:377–98. - PubMed
-
- Muto T, Hsieh SD, Sakurai Y et al Prevalence of atopic dermatitis in Japanese adults. Br J Dermatol 2003; 148:117–21. - PubMed
-
- Saeki H, Oiso N, Honma M et al Prevalence of atopic dermatitis in Japanese adults and community validation of the U.K. diagnostic criteria. J Dermatol Sci 2009; 55:140–1. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

