A Randomized, Controlled Trial of the Analytic and Diagnostic Performance of Singleton and Trio, Rapid Genome and Exome Sequencing in Ill Infants
- PMID: 31564432
- PMCID: PMC6817534
- DOI: 10.1016/j.ajhg.2019.08.009
A Randomized, Controlled Trial of the Analytic and Diagnostic Performance of Singleton and Trio, Rapid Genome and Exome Sequencing in Ill Infants
Abstract
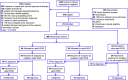
The second Newborn Sequencing in Genomic Medicine and Public Health study was a randomized, controlled trial of the effectiveness of rapid whole-genome or -exome sequencing (rWGS or rWES, respectively) in seriously ill infants with diseases of unknown etiology. Here we report comparisons of analytic and diagnostic performance. Of 1,248 ill inpatient infants, 578 (46%) had diseases of unknown etiology. 213 infants (37% of those eligible) were enrolled within 96 h of admission. 24 infants (11%) were very ill and received ultra-rapid whole-genome sequencing (urWGS). The remaining infants were randomized, 95 to rWES and 94 to rWGS. The analytic performance of rWGS was superior to rWES, including variants likely to affect protein function, and ClinVar pathogenic/likely pathogenic variants (p < 0.0001). The diagnostic performance of rWGS and rWES were similar (18 diagnoses in 94 infants [19%] versus 19 diagnoses in 95 infants [20%], respectively), as was time to result (median 11.0 versus 11.2 days, respectively). However, the proportion diagnosed by urWGS (11 of 24 [46%]) was higher than rWES/rWGS (p = 0.004) and time to result was less (median 4.6 days, p < 0.0001). The incremental diagnostic yield of reflexing to trio after negative proband analysis was 0.7% (1 of 147). In conclusion, rapid genomic sequencing can be performed as a first-tier diagnostic test in inpatient infants. urWGS had the shortest time to result, which was important in unstable infants, and those in whom a genetic diagnosis was likely to impact immediate management. Further comparison of urWGS and rWES is warranted because genomic technologies and knowledge of variant pathogenicity are evolving rapidly.
Keywords: diagnosis; genetic disease; genomic medicine; infant; intensive care unit; precision medicine; ultra-rapid whole-genome sequencing; whole-exome sequencing; whole-genome sequencing.
Copyright © 2019 American Society of Human Genetics. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
C.I.K. and P.S. are employees and shareholders of Diploid Inc., have a management relationship with Diploid Inc., and have a patent related to this work. The remaining authors declare no competing interests.
Figures


References
-
- Murphy S.L., Xu J., Kochanek K.D., Arias E. 2018. Mortality in the United States, 2017, NCHS Data Brief.https://www.ncbi.nlm.nih.gov/pubmed/30500322 - PubMed
-
- Berry M.A., Shah P.S., Brouillette R.T., Hellmann J. Predictors of mortality and length of stay for neonates admitted to children’s hospital neonatal intensive care units. J. Perinatol. 2008;28:297–302. - PubMed
-
- Malam F., Hartley T., Gillespie M.K., Armour C.M., Bariciak E., Graham G.E., Nikkel S.M., Richer J., Sawyer S.L., Boycott K.M., Dyment D.A. Benchmarking outcomes in the Neonatal Intensive Care Unit: Cytogenetic and molecular diagnostic rates in a retrospective cohort. Am. J. Med. Genet. A. 2017;173:1839–1847. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

