Stimulus- and Neural-Referred Visual Receptive Field Properties following Hemispherectomy: A Case Study Revisited
- PMID: 31565050
- PMCID: PMC6745132
- DOI: 10.1155/2019/6067871
Stimulus- and Neural-Referred Visual Receptive Field Properties following Hemispherectomy: A Case Study Revisited
Abstract
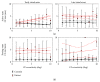
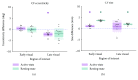
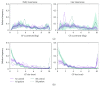
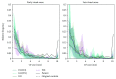
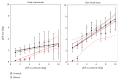
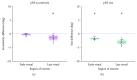
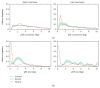
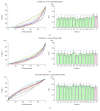
Damage to the visual system can result in (a partial) loss of vision, in response to which the visual system may functionally reorganize. Yet the timing, extent, and conditions under which this occurs are not well understood. Hence, studies in individuals with diverse congenital and acquired conditions and using various methods are needed to better understand this. In the present study, we examined the visual system of a young girl who received a hemispherectomy at the age of three and who consequently suffered from hemianopia. We did so by evaluating the corticocortical and retinocortical projections in the visual system of her remaining hemisphere. For the examination of these aspects, we analyzed the characteristics of the connective fields ("neural-referred" receptive fields) based on both resting-state (RS) and retinotopy data. The evaluation of RS data, reflecting brain activity independent from visual stimulation, is of particular interest as it is not biased by the patient's atypical visual percept. We found that, primarily when the patient was at rest, the connective fields between V1 and both early and late visual areas were larger than normal. These abnormally large connective fields could be a sign either of functional reorganization or of unmasked suppressive feedback signals that are normally masked by interhemispheric signals. Furthermore, we confirmed our previous finding of abnormal retinocortical or "stimulus-referred" projections in both early and late visual areas. More specifically, we found an enlarged foveal representation and smaller population receptive fields. These differences could also be a sign of functional reorganization or rather a reflection of the interruption visual information that travels, via the remainder of the visual pathway, from the retina to the visual cortex. To conclude, while we do find indications for relatively subtle changes in visual field map properties, we found no evidence of large-scale reorganization-even though the patient could have benefitted from this. Our work suggests that at a later developmental stage, large-scale reorganization of the visual system no longer occurs, while small-scale properties may still change to facilitate adaptive processing and viewing strategies.
Copyright © 2019 Hinke N. Halbertsma et al.
Conflict of interest statement
The authors declare no conflict of interests.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

