Accuracy of Colon Capsule Endoscopy for Colorectal Neoplasia Detection in Individuals Referred for a Screening Colonoscopy
- PMID: 31565052
- PMCID: PMC6745144
- DOI: 10.1155/2019/5975438
Accuracy of Colon Capsule Endoscopy for Colorectal Neoplasia Detection in Individuals Referred for a Screening Colonoscopy
Abstract
Backround: Capsule colonoscopy might present an alternative to colonoscopy for colorectal neoplasia screening.
Aim: To assess the accuracy of second-generation capsule colonoscopy (CCE2) for colorectal neoplasia detection compared with conventional colonoscopy (CC).
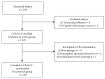
Methods: From 2011-2015, we performed a multicenter, prospective, cross-over study evaluating the use of CCE2 as a possible colorectal cancer (CRC) screening test based on the assessment of the method's characteristics (accuracy) and safety and patient acceptance of the routine. Enrolled participants fulfilled the CRC screening population criteria if they were asymptomatic, were older than 50, and had no personal or familial history of colorectal neoplasia. The primary outcome was accuracy for the detection of polyps ≥ 6 mm. Secondary outcomes were accuracy for all polyps, polyps ≥ 10 mm, adenomas ≥ 10 mm, and cancers, the quality of bowel cleansing, safety, and CCE2 acceptability by the screening population.
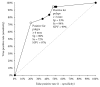
Results: A total of 236 individuals were examined; 11 patients (5%) were excluded. Therefore, 225 subjects (95%) were considered in the intention-to-screen (ITS) group. A total of 201 patients (89%) completed both examinations successfully (per protocol group). In the ITS group, polyps were diagnosed during CC in 114 subjects (51%); polyps ≥ 6 mm, polyps ≥ 10 mm, and adenomas ≥ 10 mm were diagnosed in 34 (15%), 16 (7%), and 11 (5%) patients, respectively. The sensitivity of CCE2 for polyps ≥ 6 mm, polyps ≥ 10 mm, and adenomas ≥ 10 mm was 79% (95% confidence interval (CI): 62-91%), 88% (95% CI: 62-98%), and 100% (95% CI: 72-100%), respectively.
Conclusion: Second-generation capsule colonoscopy is a safe, noninvasive, and sensitive method for colorectal neoplasia detection although CC remains the preferred method for considerable proportion of subjects. CCE2 may therefore be accepted as the primary screening test for colorectal cancer screening.
Copyright © 2019 Michal Voska et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures

References
-
- Kamangar F., Dores G. M., Anderson W. F. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. Journal of Clinical Oncology. 2006;24(14):2137–2150. doi: 10.1200/JCO.2005.05.2308. - DOI - PubMed
LinkOut - more resources
Full Text Sources

