Histone Acetyltransferase-Dependent Pathways Mediate Upregulation of NADPH Oxidase 5 in Human Macrophages under Inflammatory Conditions: A Potential Mechanism of Reactive Oxygen Species Overproduction in Atherosclerosis
- PMID: 31565149
- PMCID: PMC6745143
- DOI: 10.1155/2019/3201062
Histone Acetyltransferase-Dependent Pathways Mediate Upregulation of NADPH Oxidase 5 in Human Macrophages under Inflammatory Conditions: A Potential Mechanism of Reactive Oxygen Species Overproduction in Atherosclerosis
Abstract
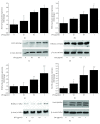
Histone acetylation plays a major role in epigenetic regulation of gene expression. Monocyte-derived macrophages express functional NADPH oxidase 5 (Nox5) that contributes to oxidative stress in atherogenesis. The mechanisms of Nox5 regulation are not entirely elucidated. The aim of this study was to investigate the expression pattern of key histone acetyltransferase subtypes (p300, HAT1) in human atherosclerosis and to determine their role in mediating the upregulation of Nox5 in macrophages under inflammatory conditions. Human nonatherosclerotic and atherosclerotic tissue samples were collected in order to determine the expression of p300 and HAT1 isoforms, H3K27ac, and Nox5. In vitro determinations were done on human macrophages exposed to lipopolysaccharide in the absence or presence of histone acetyltransferase inhibitors. Western blot, immunohistochemistry, immunofluorescence, real-time PCR, transfection, and chromatin immunoprecipitation assay were employed. The protein levels of p300 and HAT1 isoforms, H3K27ac, and Nox5 were found significantly elevated in human atherosclerotic specimens. Immunohistochemistry/immunofluorescence staining revealed that p300, HAT1, H3K27ac, H3K9ac, and Nox5 proteins were colocalized in the area of CD45+/CD68+ immune cells and lipid-rich deposits within human atherosclerotic plaques. Lipopolysaccharide induced the levels of HAT1, H3K27ac, H3K9ac, and Nox5 and the recruitment of p300 and HAT1 at the sites of active transcription within Nox5 gene promoter in cultured human macrophages. Pharmacological inhibition of histone acetyltransferase significantly reduced the Nox5 gene and protein expression in lipopolysaccharide-challenged macrophages. The overexpression of p300 or HAT1 enhanced the Nox5 gene promoter activity. The histone acetyltransferase system is altered in human atherosclerosis. Under inflammatory conditions, HAT subtypes control Nox5 overexpression in cultured human macrophages. The data suggest the existence of a new epigenetic mechanism underlying oxidative stress in atherosclerosis.
Copyright © 2019 Mihaela-Loredana Vlad et al.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








References
-
- Manea A., Simionescu M. Nox enzymes and oxidative stress in atherosclerosis. Frontiers in Bioscience (Scholar Edition) 2012;4:651–670. - PubMed
-
- Lee C. F., Qiao M., Schröder K., Zhao Q., Asmis R. Nox4 is a novel inducible source of reactive oxygen species in monocytes and macrophages and mediates oxidized low density lipoprotein-induced macrophage death. Circulation Research. 2010;106(9):1489–1497. doi: 10.1161/CIRCRESAHA.109.215392. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

