Efficacy, immunogenicity and safety of vaccination in adult patients with autoimmune inflammatory rheumatic diseases: a systematic literature review for the 2019 update of EULAR recommendations
- PMID: 31565247
- PMCID: PMC6744079
- DOI: 10.1136/rmdopen-2019-001035
Efficacy, immunogenicity and safety of vaccination in adult patients with autoimmune inflammatory rheumatic diseases: a systematic literature review for the 2019 update of EULAR recommendations
Abstract
Aim: To present a systematic literature review (SLR) on efficacy, immunogenicity and safety of vaccination in adult patients with autoimmune inflammatory rheumatic diseases (AIIRD), aiming to provide a basis for updating the EULAR evidence-based recommendations.
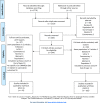
Methods: An SLR was performed according to the standard operating procedures for EULAR-endorsed recommendations. Outcome was determined by efficacy, immunogenicity and safety of vaccination in adult patients with AIIRD, including those receiving immunomodulating therapy. Furthermore, a search was performed on the effect of vaccinating household members of patients with AIIRD on the occurrence of vaccine-preventable infections in patients and their household members (including newborns). The literature search was performed using Medline, Embase and the Cochrane Library (October 2009 to August 2018).
Results: While most investigated vaccines were efficacious and/or immunogenic in patients with AIIRD, some were less efficacious than in healthy control subjects, and/or in patients receiving immunosuppressive agents. Adverse events of vaccination were generally mild and the rates were comparable to those in healthy persons. Vaccination did not seem to lead to an increase in activity of the underlying AIIRD, but insufficient power of most studies precluded arriving at definite conclusions. The number of studies investigating clinical efficacy of vaccination is still limited. No studies on the effect of vaccinating household members of patients with AIIRD were retrieved.
Conclusion: Evidence on efficacy, immunogenicity and safety of vaccination in patients with AIIRD was systematically reviewed to provide a basis for updated recommendations.
Keywords: autoimmune diseases; infections; vaccination.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: KW reports personal fees from Pfizer, during the conduct of the study; grants and personal fees from Biotest, CSL Behring, personal fees from LFB, Grifols, Baxter, Roche, Octapharma, grants from BMS, outside the submitted work. AM reports grants and personal fees from ABBVIE, Pfizer, BMS, UCB, MERCK, during the conduct of the study. MD reports grants and personal fees from PFIZER, ABBVIE, UCB, NOVARTIS, LILLY, MERCK, ROCHE, grants from BMS, outside the submitted work. OE reports grants and personal fees from Pfizer, Abbvie, Roche, Janssen, personal fees from Novartis and Lilly. All other authors have no conflicts of interests to declare.
Figures
Similar articles
-
Incidence and prevalence of vaccine preventable infections in adult patients with autoimmune inflammatory rheumatic diseases (AIIRD): a systemic literature review informing the 2019 update of the EULAR recommendations for vaccination in adult patients with AIIRD.RMD Open. 2019 Sep 19;5(2):e001041. doi: 10.1136/rmdopen-2019-001041. eCollection 2019. RMD Open. 2019. PMID: 31673420 Free PMC article.
-
Vaccination in adult patients with auto-immune inflammatory rheumatic diseases: a systematic literature review for the European League Against Rheumatism evidence-based recommendations for vaccination in adult patients with auto-immune inflammatory rheumatic diseases.Autoimmun Rev. 2011 Apr;10(6):341-52. doi: 10.1016/j.autrev.2010.12.003. Epub 2010 Dec 20. Autoimmun Rev. 2011. PMID: 21182987
-
2019 update of EULAR recommendations for vaccination in adult patients with autoimmune inflammatory rheumatic diseases.Ann Rheum Dis. 2020 Jan;79(1):39-52. doi: 10.1136/annrheumdis-2019-215882. Epub 2019 Aug 14. Ann Rheum Dis. 2020. PMID: 31413005
-
EULAR recommendations for vaccination in adult patients with autoimmune inflammatory rheumatic diseases.Ann Rheum Dis. 2011 Mar;70(3):414-22. doi: 10.1136/ard.2010.137216. Epub 2010 Dec 3. Ann Rheum Dis. 2011. PMID: 21131643
-
Vaccination recommendations for adult patients with autoimmune inflammatory rheumatic diseases.Swiss Med Wkly. 2015 Jul 28;145:w14159. doi: 10.4414/smw.2015.14159. eCollection 2015. Swiss Med Wkly. 2015. PMID: 26218860
Cited by
-
Older Age, a High Titre of Neutralising Antibodies and Therapy with Conventional DMARDs Are Associated with Protection from Breakthrough Infection in Rheumatoid Arthritis Patients after the Booster Dose of Anti-SARS-CoV-2 Vaccine.Vaccines (Basel). 2023 Nov 2;11(11):1684. doi: 10.3390/vaccines11111684. Vaccines (Basel). 2023. PMID: 38006015 Free PMC article.
-
Humoral and cellular immunity in patients with rare autoimmune rheumatic diseases following SARS-CoV-2 vaccination.Rheumatology (Oxford). 2023 Jun 1;62(6):2294-2303. doi: 10.1093/rheumatology/keac574. Rheumatology (Oxford). 2023. PMID: 36250898 Free PMC article.
-
Association of COVID-19 antigenicity with the development of antineutrophilic cytoplasmic antibody vasculitis.Respirol Case Rep. 2021 Dec 27;10(1):e0894. doi: 10.1002/rcr2.894. eCollection 2022 Jan. Respirol Case Rep. 2021. PMID: 34992785 Free PMC article.
-
Kinetics of humoral immune response and severity of infection after three doses of SARS-CoV-2 mRNA vaccine in a large cohort of kidney transplant recipients.J Nephrol. 2023 Jul;36(6):1663-1671. doi: 10.1007/s40620-023-01650-8. Epub 2023 Jul 17. J Nephrol. 2023. PMID: 37458909 Free PMC article.
-
Systemic Sclerosis and Vaccinations: A Register-Based Cohort Study about Seasonal Influenza and Streptococcus pneumoniae Vaccination Rate and Uptake from Liguria Regional Center, Northwest Italy.Vaccines (Basel). 2020 Apr 28;8(2):204. doi: 10.3390/vaccines8020204. Vaccines (Basel). 2020. PMID: 32354027 Free PMC article.
References
-
- Furer V, Rondaan C, Heijstek M, et al. . Incidence and prevalence of vaccine preventable infections in adult patients with autoimmune inflammatory rheumatic diseases (AIIRD): a systemic literature review Informing the 2019 update of the EULAR recommendations for vaccination in adult patients with AIIRD. RMD Open 2019. - PMC - PubMed
-
- Phillips B, Ball C, Sackett D, et al. . Last update by Howick J. Oxford centre for evidence-based medicine – levels of evidence (March 2009), 2018.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical