Vanadium Dioxide Nanocoating Induces Tumor Cell Death through Mitochondrial Electron Transport Chain Interruption
- PMID: 31565366
- PMCID: PMC6436600
- DOI: 10.1002/gch2.201800058
Vanadium Dioxide Nanocoating Induces Tumor Cell Death through Mitochondrial Electron Transport Chain Interruption
Abstract
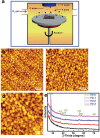
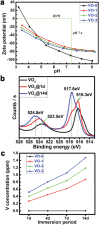
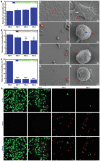
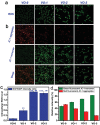
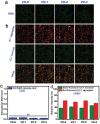
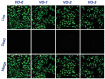
A biomaterials surface enabling the induction of tumor cell death is particularly desirable for implantable biomedical devices that directly contact tumor tissues. However, this specific antitumor feature is rarely found. Consequently, an antitumor-cell nanocoating comprised of vanadium dioxide (VO2) prepared by customized reactive magnetron sputtering has been proposed, and its antitumor-growth capability has been demonstrated using human cholangiocarcinoma cells. The results reveal that the VO2 nanocoating is able to interrupt the mitochondrial electron transport chain and then elevate the intracellular reactive oxygen species levels, leading to the collapse of the mitochondrial membrane potential and the destruction of cell redox homeostasis. Indeed, this chain reaction can effectively trigger oxidative damage in the cholangiocarcinoma cells. Additionally, this study has provided new insights into designing a tumor-cell-inhibited biomaterial surface, which is modulated by the mechanism of mitochondria-targeting tumor cell death.
Keywords: anticancer; charge transfer; functional coatings and films; mitochondria; vanadium dioxide.
© 2018 The Authors. Published by WILEY‐VCH Verlag GmbH & Co. KGaA, Weinheim.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- a) Hanker J., Giammara B., Science 1988, 242, 885; - PubMed
- b) Tang R., Moyano D. F., Subramani C., Yan B., Jeoung E., Tonga G. Y., Duncan B., Yeh Y.‐C., Jiang Z., Kim C., Rotello V. M., Adv. Mater. 2014, 26, 3310; - PMC - PubMed
- c) Bowen P. K., Drelich J., Goldman J., Adv. Mater. 2013, 25, 2577; - PubMed
- d) Wang H., Kwok D. T. K., Xu M., Shi H., Wu Z., Zhang W., Chu P. K., Adv. Mater. 2012, 24, 3315; - PubMed
- e) Banerjee I., Pangule R. C., Kane R. S., Adv. Mater. 2011, 23, 690; - PubMed
- f) Hu Y., Cai K. Y., Luo Z., Jandt K. D., Adv. Mater. 2010, 22, 4146. - PubMed
-
- a) Ma H., Luo J., Sun Z., Xia L., Shi M., Liu M., Chang J., Wu C., Biomaterials 2016, 111, 138; - PubMed
- b) Zhang M., Cheng H., Gong Z., Zhang J., Liu X., Wang B., Ban L., Zeng Y., Zhu Z., Adv. Funct. Mater. 2017, 27, 1703932.
-
- a) Ma H., Jiang C., Zhai D., Luo Y., Chen Y., Lv F., Yi Z., Deng Y., Wang J., Chang J., Wu C., Adv. Funct. Mater. 2016, 26, 1197;
- b) Wang X., Li T., Ma H., Zhai D., Jiang C., Chang J., Wang J., Wu C., NPG Asia Mater. 2017, 9, e376.
LinkOut - more resources
Full Text Sources

