Geriatric Research Policy: Japan Clinical Oncology Group (JCOG) policy
- PMID: 31565730
- PMCID: PMC6886463
- DOI: 10.1093/jjco/hyz093
Geriatric Research Policy: Japan Clinical Oncology Group (JCOG) policy
Abstract
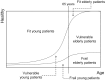
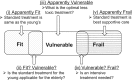
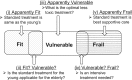
Due to the rapid aging of Japan's population, clinical research focusing on older patients with cancer is urgently needed. The Japan Clinical Oncology Group (JCOG) has conducted several such clinical trials, but there has been no formal policy for geriatric research. We have therefore established a JCOG policy for geriatric cancer research. We defined the patient selection policy based on treatment tolerance and chronological age. Older patients are categorized into three conceptual groups: 'fit patients' who can undergo the same standard treatment given to younger patients, 'frail patients' for whom best supportive or palliative care is indicated and 'vulnerable patients' who fall between the fit and frail categories. Unmet needs often exist for vulnerable patients. The policy recommends that study endpoints include not only survival but also other endpoints such as physical and cognitive function because the objective of therapy in older patients is not only extended life expectancy but also maintenance of the patient's general condition. In this viewpoint, co-primary or composite endpoints that incorporate geriatric assessment in the study design are often applicable. Study design will differ depending on the study population, clinical question, and treatment. Even for older patients, a randomized clinical trial is still the gold standard when the clinical question asks which treatment is better. An observational study of a broader population is applicable for investigating actual conditions of older patients. This JCOG Geriatric Research Policy includes several practical solutions for various issues in geriatric research. We plan to revise this policy periodically to guide future geriatric research.
Keywords: JCOG; endpoint; geriatric; geriatric assessment; study designs.
© The Author(s) 2019. Published by Oxford University Press.
Figures
Similar articles
-
[Elderly Glioma].No Shinkei Geka. 2021 May;49(3):647-659. doi: 10.11477/mf.1436204439. No Shinkei Geka. 2021. PMID: 34092571 Japanese.
-
[Geriatric Oncology].Gan To Kagaku Ryoho. 2017 Feb;44(2):97-101. Gan To Kagaku Ryoho. 2017. PMID: 28223666 Review. Japanese.
-
Patient-reported outcome and quality of life research policy: Japan Clinical Oncology Group (JCOG) policy.Jpn J Clin Oncol. 2023 Mar 7;53(3):195-202. doi: 10.1093/jjco/hyad007. Jpn J Clin Oncol. 2023. PMID: 36702740 Free PMC article.
-
Study risk assessment of Japan Clinical Oncology Group (JCOG) clinical trials using the European Organisation for Research and Treatment of Cancer (EORTC) study risk calculator.Jpn J Clin Oncol. 2019 Aug 1;49(8):727-733. doi: 10.1093/jjco/hyz050. Jpn J Clin Oncol. 2019. PMID: 31329908
-
Geriatric oncology research to improve clinical care.Nat Rev Clin Oncol. 2012 Oct;9(10):571-8. doi: 10.1038/nrclinonc.2012.125. Epub 2012 Jul 24. Nat Rev Clin Oncol. 2012. PMID: 22825377 Free PMC article. Review.
Cited by
-
A multicenter prospective observational study for health assessment questionnaires EQ-5D-5L and G8 in unresectable advanced pancreatic cancer treated with first-line gemcitabine plus nab-paclitaxel therapy.Int J Clin Oncol. 2025 Apr;30(4):738-748. doi: 10.1007/s10147-025-02717-1. Epub 2025 Feb 27. Int J Clin Oncol. 2025. PMID: 40011378
-
Challenges in Geriatric Oncology-A Surgeon's Perspective.Curr Oncol. 2022 Jan 29;29(2):659-674. doi: 10.3390/curroncol29020058. Curr Oncol. 2022. PMID: 35200558 Free PMC article. Review.
-
Practical management of older adults with cancer: geriatric oncology in Japan.Jpn J Clin Oncol. 2022 Oct 6;52(10):1073-1081. doi: 10.1093/jjco/hyac118. Jpn J Clin Oncol. 2022. PMID: 35863011 Free PMC article. Review.
-
Geriatric assessment and management with question prompt list using a web-based application for elderly patients with cancer (MAPLE) to communicate ageing-related concerns: J-SUPPORT 2101 study protocol for a multicentre, parallel group, randomised controlled trial.BMJ Open. 2022 Sep 26;12(9):e063445. doi: 10.1136/bmjopen-2022-063445. BMJ Open. 2022. PMID: 36167377 Free PMC article.
-
The landscape of immune therapy in vulnerable patients with advanced non-small cell lung cancer: a narrative review.Transl Lung Cancer Res. 2023 Nov 30;12(11):2310-2321. doi: 10.21037/tlcr-23-581. Epub 2023 Nov 21. Transl Lung Cancer Res. 2023. PMID: 38090528 Free PMC article. Review.
References
-
- Muramatsu N, Akiyama H. Japan: super-aging society preparing for the future. The Gerontologist 2011;51:425–32. - PubMed
-
- CANCER STATISTICS IN JAPAN ‘17. https://ganjoho.jp/en/professional/statistics/brochure/2017_en.html.
-
- World Population Ageing 2017. https://www.un.org/en/development/desa/population/theme/ageing/WPA2017.asp.
-
- CANCER RESEARCH UK.
-
- Hutchins LF, Unger JM, Crowley JJ, Coltman CA Jr., Albain KS. Underrepresentation of patients 65 years of age or older in cancer-treatment trials. N Engl J Med 1999;341:2061–7. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources