Smoking reduction interventions for smoking cessation
- PMID: 31565800
- PMCID: PMC6953262
- DOI: 10.1002/14651858.CD013183.pub2
Smoking reduction interventions for smoking cessation
Abstract
Background: The standard way most people are advised to stop smoking is by quitting abruptly on a designated quit day. However, many people who smoke have tried to quit many times and may like to try an alternative method. Reducing smoking behaviour before quitting could be an alternative approach to cessation. However, before this method can be recommended it is important to ensure that abrupt quitting is not more effective than reducing to quit, and to determine whether there are ways to optimise reduction methods to increase the chances of cessation.
Objectives: To assess the effect of reduction-to-quit interventions on long-term smoking cessation.
Search methods: We searched the Cochrane Tobacco Addiction Group Specialised Register, MEDLINE, Embase and PsycINFO for studies, using the terms: cold turkey, schedul*, cut* down, cut-down, gradual*, abrupt*, fading, reduc*, taper*, controlled smoking and smoking reduction. We also searched trial registries to identify unpublished studies. Date of the most recent search: 29 October 2018.
Selection criteria: Randomised controlled trials in which people who smoked were advised to reduce their smoking consumption before quitting smoking altogether in at least one trial arm. This advice could be delivered using self-help materials or behavioural support, and provided alongside smoking cessation pharmacotherapies or not. We excluded trials that did not assess cessation as an outcome, with follow-up of less than six months, where participants spontaneously reduced without being advised to do so, where the goal of reduction was not to quit altogether, or where participants were advised to switch to cigarettes with lower nicotine levels without reducing the amount of cigarettes smoked or the length of time spent smoking. We also excluded trials carried out in pregnant women.
Data collection and analysis: We followed standard Cochrane methods. Smoking cessation was measured after at least six months, using the most rigorous definition available, on an intention-to-treat basis. We calculated risk ratios (RRs) and 95% confidence intervals (CIs) for smoking cessation for each study, where possible. We grouped eligible studies according to the type of comparison (no smoking cessation treatment, abrupt quitting interventions, and other reduction-to-quit interventions) and carried out meta-analyses where appropriate, using a Mantel-Haenszel random-effects model. We also extracted data on quit attempts, pre-quit smoking reduction, adverse events (AEs), serious adverse events (SAEs) and nicotine withdrawal symptoms, and meta-analysed these where sufficient data were available.
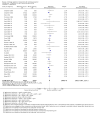
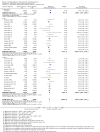
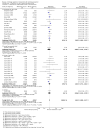
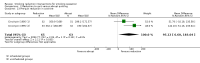
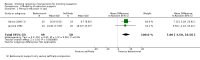
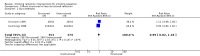
Main results: We identified 51 trials with 22,509 participants. Most recruited adults from the community using media or local advertising. People enrolled in the studies typically smoked an average of 23 cigarettes a day. We judged 18 of the studies to be at high risk of bias, but restricting the analysis only to the five studies at low or to the 28 studies at unclear risk of bias did not significantly alter results.We identified very low-certainty evidence, limited by risk of bias, inconsistency and imprecision, comparing the effect of reduction-to-quit interventions with no treatment on cessation rates (RR 1.74, 95% CI 0.90 to 3.38; I2 = 45%; 6 studies, 1599 participants). However, when comparing reduction-to-quit interventions with abrupt quitting (standard care) we found evidence that neither approach resulted in superior quit rates (RR 1. 01, 95% CI 0.87 to 1.17; I2 = 29%; 22 studies, 9219 participants). We judged this estimate to be of moderate certainty, due to imprecision. Subgroup analysis provided some evidence (P = 0.01, I2 = 77%) that reduction-to-quit interventions may result in more favourable quit rates than abrupt quitting if varenicline is used as a reduction aid. Our analysis comparing reduction using pharmacotherapy with reduction alone found low-certainty evidence, limited by inconsistency and imprecision, that reduction aided by pharmacotherapy resulted in higher quit rates (RR 1. 68, 95% CI 1.09 to 2.58; I2 = 78%; 11 studies, 8636 participants). However, a significant subgroup analysis (P < 0.001, I2 = 80% for subgroup differences) suggests that this may only be true when fast-acting NRT or varenicline are used (both moderate-certainty evidence) and not when nicotine patch, combination NRT or bupropion are used as an aid (all low- or very low-quality evidence). More evidence is likely to change the interpretation of the latter effects.Although there was some evidence from within-study comparisons that behavioural support for reduction to quit resulted in higher quit rates than self-help resources alone, the relative efficacy of various other characteristics of reduction-to-quit interventions investigated through within- and between-study comparisons did not provide any evidence that they enhanced the success of reduction-to-quit interventions. Pre-quit AEs, SAEs and nicotine withdrawal symptoms were measured variably and infrequently across studies. There was some evidence that AEs occurred more frequently in studies that compared reduction using pharmacotherapy versus no pharmacotherapy; however, the AEs reported were mild and usual symptoms associated with NRT use. There was no clear evidence that the number of people reporting SAEs, or changes in withdrawal symptoms, differed between trial arms.
Authors' conclusions: There is moderate-certainty evidence that neither reduction-to-quit nor abrupt quitting interventions result in superior long-term quit rates when compared with one another. Evidence comparing the efficacy of reduction-to-quit interventions with no treatment was inconclusive and of low certainty. There is also low-certainty evidence to suggest that reduction-to-quit interventions may be more effective when pharmacotherapy is used as an aid, particularly fast-acting NRT or varenicline (moderate-certainty evidence). Evidence for any adverse effects of reduction-to-quit interventions was sparse, but available data suggested no excess of pre-quit SAEs or withdrawal symptoms. We downgraded the evidence across comparisons due to risk of bias, inconsistency and imprecision. Future research should aim to match any additional components of multicomponent reduction-to-quit interventions across study arms, so that the effect of reduction can be isolated. In particular, well-conducted, adequately-powered studies should focus on investigating the most effective features of reduction-to-quit interventions to maximise cessation rates.
Conflict of interest statement
BH has no known conflicts of interest.
EK has no known conflicts of interest. EK was an author of one included study (Klemperer 2017). They did not carry out data extraction or risk of bias assessment for this study.
JMOM has no known conflicts of interest.
NL has no known conflicts of interest. NL was an author of one included study (Lindson‐Hawley 2016b). They did not carry out data extraction or risk of bias assessment for this study.
PA has no known conflicts of interest. PA was an author of two included studies (Farley 2017; Lindson‐Hawley 2016b) They did not carry out data extraction or risk of bias assessment for those studies.
Figures



































Update of
- doi: 10.1002/14651858.CD013183
References
References to studies included in this review
Blevins 2016 {published data only}
Bolliger 2000a {published data only}
-
- Bolliger CT, Zellweger JP, Danielsson T, Biljon X, Robidou A, Westin A, et al. Influence of long‐term smoking reduction on health risk markers and quality of life. Nicotine & Tobacco Research 2002;4(4):433‐9. - PubMed
Brockway 1977 {published data only}
-
- Brockway BS, Kleinmann G, Edleson J, Gruenewald K. Non aversive procedures and their effect on cigarette smoking. Addictive Behaviors 1977;2(2‐3):121‐8. - PubMed
Caldwell 2016 {published data only}
-
- ACTRN12609000481279. Nicotine inhaler plus nicotine patch for smoking cessation [Pulmonary nicotine inhaler plus nicotine transdermal patch for 6‐month smoking cessation in heavy smokers]. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=308032 (first received 18 June 2009).
-
- Caldwell BO, Crane J. Combination nicotine metered dose inhaler and nicotine patch for smoking cessation: a randomized controlled trial. Nicotine & Tobacco Research 2016;18(10):1944‐51. - PubMed
Carpenter 2003 {published data only}
-
- Carpenter MJ, Hughes JR, Keely JP. Effect of smoking reduction on later cessation: a pilot experimental study. Nicotine & Tobacco Research 2003;5(2):155‐62. - PubMed
Carpenter 2004 {published data only}
-
- Carpenter MJ, Hughes JR, Solomon LJ, Callas PW. Both smoking reduction with nicotine replacement therapy and motivational advice increase future cessation among smokers unmotivated to quit. Journal of Consulting and Clinical Psychology 2004;72(3):371‐81. - PubMed
-
- Hughes JR, Carpenter MJ. Smoking reduction with NRT increases, not decreases, future cessation. Society for Research on Nicotine and Tobacco 5th European Meeting; 2003 Nov 20‐22; Padua, Italy. 2003.
Chan 2011 {published data only}
-
- Lam T‐H, Chan SS, Abdullah AS, Taam Wong V, Chan AY, Hedley AJ. Smoking reduction intervention for smokers not willing to quit smoking: A randomised controlled trial. Hong Kong Medical Journal 2012;18(3):4‐8. - PubMed
-
- NCT00563329. Smoking reduction intervention for smokers not willing to quit smoking: a randomised control trial. clinicaltrials.gov/ct2/show/NCT00563329 (first received 26 Nov 2007).
Cinciripini 1995 {published data only}
-
- Cinciripini PM, Lapitsky L, Seay S, Wallfisch A, Kitchens K, Vunakis H. The effects of smoking schedules on cessation outcome: can we improve on common methods of gradual and abrupt nicotine withdrawal?. Journal of Consulting and Clinical Psychology 1995;63(3):388‐99. - PubMed
-
- Cinciripini PM, Wetter DW, McClure JB. Scheduled reduced smoking: effects on smoking abstinence and potential mechanisms of action. Addictive Behaviors 1997;22(6):759‐67. - PubMed
Cinciripini 2006 {published data only}
-
- Cinciripini PM, Lam C, Blalock JA, Robinson J, Wetter DE, Baile W. Does scheduled reduced smoking have a place among smoking cessation treatments?. Society for Research on Nicotine and Tobacco 12th Annual Meeting; 2006 Feb 15‐18; Orlando, USA. 2006:14.
Cook 2016 {published data only}
-
- NCT01122238. Identifying treatments to motivate smokers to quit. clinicaltrials.gov/ct2/show/NCT01122238 (first received 13 May 2010).
Cummings 1988 {published data only}
-
- Cummings KM, Emont SL, Jaen C, Sciandra R. Format and quitting instructions as factors influencing the impact of a self‐administered quit smoking program. Health Education Quarterly 1988;15(2):199‐216. - PubMed
Curry 1988 {published data only}
-
- Curry SJ, Marlatt GA, Gordon J, Baer JS. A comparison of alternative theoretical approaches to smoking cessation and relapse. Health Psychology 1988;7(6):545‐56. - PubMed
Dooley 1992 {published data only}
-
- Dooley RT, Halford WK. A comparison of relapse prevention with nicotine gum or nicotine fading in modification of smoking. Australian Psychologist 1992;27(3):186‐91.
Ebbert 2015 {published data only}
-
- Fagerström K. Varenicline can help smokers to stop smoking by gradual reduction. BMJ Evidence‐Based Medicine 2015;20(4):133. - PubMed
-
- Nakamura M, Abe M, Ohkura M, Treadow J, Yu CR, Park PW. Efficacy of varenicline for cigarette reduction before quitting in Japanese smokers: a subpopulation analysis of the reduce to quit trial. Clinical Therapeutics 2017;39(4):863‐72. - PubMed
Etter 2002 {published data only}
-
- Dar R, Stronguin F, Etter JF. Assigned versus perceived placebo effects in nicotine replacement therapy for smoking reduction in Swiss smokers. Journal of Consulting and Clinical Psychology 2005;73(2):350‐3. - PubMed
-
- Etter JF. Reduction of exposure to cigarette smoke: a randomized trial. 11th World Conference on Tobacco or Health; 2000 Aug 6‐11; Chicago, USA. 2000:159.
-
- Etter JF, Laszlo E. Postintervention effect of nicotine replacement therapy for smoking reduction: a randomized trial with a 5‐year follow‐up. Journal of Clinical Psychopharmacology 2007;27(2):151‐5. - PubMed
-
- Etter JF, Laszlo E, Perneger TV. Postintervention effect of nicotine replacement therapy on smoking reduction in smokers who are unwilling to quit: randomized trial. Journal of Clinical Psychopharmacology 2004;24(2):174‐9. - PubMed
-
- Etter JF, Laszlo E, Zellweger JP, Perrot C, Perneger TV. Nicotine replacement to reduce cigarette consumption in smokers who are unwilling to quit: a randomized trial. Society for Research on Nicotine and Tobacco 8th Annual Meeting; 2002 Feb 20‐23; Savannah, USA. 2002:27. [PA6‐3] - PubMed
Etter 2009 {published data only}
-
- Etter JF, Huguelet P, Perneger TV, Cornuz J. Nicotine gum treatment before smoking cessation: a randomized trial. Archives of Internal Medicine 2009;169(11):1028‐34. - PubMed
Farley 2017 {published data only}
Flaxman 1978 {published data only}
-
- Flaxman J. Gradual and abrupt quitting strategies and a successful self‐control technique for smoking modification [thesis]. USA: Dissertation Abstracts International, 1975.
-
- Flaxman J. Quitting smoking now or later: Gradual, abrupt, immediate, and delayed quitting. Behavior Therapy 1978;9(2):260‐70.
Garcia 2000 {published data only}
-
- Garcia MP, Becona E. Evaluation of the amount of therapist contact in a smoking cessation program. Spanish Journal of Psychology 2000;3(1):28‐36. - PubMed
Gariti 2004 {published data only}
-
- Gariti P, Alterman AI, Lynch KG, Kampman K, Whittingham T. Adding a nicotine blocking agent to cigarette tapering. Journal of Substance Abuse Treatment 2004;27(1):17‐25. - PubMed
Gil Roales‐Nieto 1992a {published data only}
-
- Gil Roales‐Nieto J, Parra A. Efficiency of a self‐control program for the treatment of smoking behaviour: Different effects in two strategies, one with an immediate quitting and other with a gradual quitting [Eficacia de un programa de autocontrol para el tratamiento del tabaquismo: Efectos diferenciales de dos estrategias de retirada de y reduccion]. Analisis y Modificacion de Conducta 1992;18(59):329‐44.
Glasgow 1978 {published data only (unpublished sought but not used)}
-
- Glasgow RE. Effects of a self‐control manual, rapid smoking, and amount of therapist contact on smoking reduction. Journal of Consulting and Clinical Psychology 1978;46(6):1439‐47. - PubMed
Glasgow 1989 {published data only}
-
- Glasgow RE, Morray K, Lichtenstein E. Controlled smoking versus abstinence as a treatment goal: The hopes and fears may be unfounded. Behavior Therapy 1989;20(1):77‐91.
Glasgow 2009a {published data only}
-
- Glasgow RE, Gaglio B, Estabrooks PA, Marcus AC, Ritzwoller DP, Smith TL, et al. Long‐term results of a smoking reduction program. Medical Care 2009;47(1):115‐20. - PubMed
Gunther 1992 {published data only}
-
- Gunther V, Gritsch S, Meise U. Smoking cessation ‐ gradual or sudden stopping?. Drug and Alcohol Dependence 1992;29(3):231‐6. - PubMed
Hanson 2008 {published data only}
-
- Hanson K, Zylla E, Allen S, Avery G. Harm reduction: an intervention for adolescent smokers. Society for Research on Nicotine and Tobacco 12th Annual Meeting; 2006 Feb 15‐18; Orlando, USA. 2006:4. [SYM3B]
-
- NCT00158171. Effectiveness of various smoking cessation therapies in reducing smoking in adolescents ‐ 1. clinicaltrials.gov/ct2/show/NCT00158171 (first received 12 September 2005).
Hao 2017 {published data only}
-
- Hao R, Zhou JP, Ni L, Li QY, Shi GC. Smoking abstinence rate and its associated factors between abrupt and gradual smoking cessation. Chinese Journal of Tuberculosis and Respiratory Diseases 2017;40(12):898‐902. - PubMed
Hatsukami 2004 {published data only}
-
- Hatsukami DK, Rennard S, Patel MK, Kotlyar M, Malcolm R, Nides MA, et al. Effects of sustained‐release bupropion among persons interested in reducing but not quitting smoking. American Journal of Medicine 2004;116(3):151‐7. - PubMed
-
- Rennard S, Hatsukami D, Malcolm RE, Patel MK, Jamerson BD, Dozier G. Zyban (bupropion HCL SR) vs placebo as an aid to smoking reduction among smokers unwilling and unable to quit smoking. Society for Research on Nicotine and Tobacco 7th Annual Meeting; 2001 Mar 20‐23; Seattle, USA. 2001:117. [PO4 77]
Haustein 2002 {published data only}
-
- Haustein KO, Batra A. A double‐blind, randomized, placebo‐controlled multicenter trial of a nicotine chewing gum in smoking reduction. Erlangen, Germany: Pharmacia; 2002 November Report no. 980‐CHC‐9021‐0031. [CHC‐9021/PMG0001]
-
- Haustein KO, Batra A, Landfeldt B, Westin A. The effect of short‐term or long‐term reduction on smoking cessation; results from a placebo controlled smoking reduction study with the nicotine gum. Nicotine & Tobacco Research 2003;5(2):278.
Ho 2018 {published data only}
-
- Ho KY, Li WH, Wang MP, Lam KK, Lam TH, Chan SS. Comparison of two approaches in achieving smoking abstinence among patients in an outpatient clinic: a Phase 2 randomized controlled trial. Patient Education and Counseling 2018;101(5):885‐93. - PubMed
Hughes 2010 {published data only}
-
- NCT00297492. Gradual vs. abrupt cessation treatment for smoking. clinicaltrials.gov/ct2/show/NCT00297492 (first received 28 Feb 2006).
Jerome 1992 {published data only}
-
- Jerome A, Perrone R, Kalfus G. Computer‐assisted smoking treatment ‐ a controlled evaluation and long‐term follow‐up. Journal of Advancement in Medicine 1992;5(1):29‐41.
Jerome 1999a {published data only}
-
- Jerome A, Behar A. Computerized, scheduled gradual reduction for smoking cessation: a randomized outcome study with 12‐month follow‐up. Personal communication 1999.
-
- Jerome A, Behar A, Boyd NR. Computerized scheduled gradual reduction for smoking cessation: a randomized work‐site outcome study with 12‐month follow‐up. Unpublished trial report.
Joseph 2008 {published data only}
-
- Joseph AM, Bliss RL, Zhao F, Lando H. Predictors of smoking reduction without formal intervention. Nicotine & Tobacco Research 2005;7(2):277‐82. - PubMed
Klemperer 2017 {published data only}
-
- Klemperer EM, Hughes JH. Reduction in cigarettes per day prospectively predicts quit attempts and cessation in smokers who are not ready to quit. Drug and Alcohol Dependence 2017;171:e107. [DOI: ]
-
- NCT01866722. Not quite ready to quit. clinicaltrials.gov/ct2/show/NCT01866722 (first received 31 May 2013).
Kralikova 2009 {published data only}
-
- Kralikova E, Kozak J, Rasmussen T, Cort N. Smoking reduction or cessation with nicotine gum or inhaler. Society for Research on Nicotine and Tobacco 7th Annual Meeting; 2001 Mar 20‐23; Seattle, USA. 2001:118. [PO4 80]
-
- Kralikova E, Kozak J, Rasmussen T, Cort N. The clinical benefits of NRT‐supported smoking reduction. Nicotine & Tobacco Research 2002;4(2):243.
Lindson‐Hawley 2016b {published data only}
-
- ISRCTN22526020. Rapid reduction versus abrupt quitting for smokers who want to stop soon. www.isrctn.com/ISRCTN22526020 (first received 14 October 2008).
-
- Lindson‐Hawley N, Banting M, West R, Michie S, Shinkins B, Aveyard P. Gradual versus abrupt smoking cessation: a randomized, controlled noninferiority trial. Annals of Internal Medicine 2016;164(9):585‐92. - PubMed
-
- West R, Lindson‐Hawley N, Aveyard P. Gradual versus abrupt smoking cessation. Annals of Internal Medicine 2016;165(10):742. [DOI: ] - PubMed
Malott 1984 {published data only}
NCT00158158 {published data only}
-
- NCT00158158. Effectiveness of reducing smoking in facilitating smoking cessation in adolescents ‐ 2. clinicaltrials.gov/ct2/show/NCT00158158 (first received 12 September 2005).
Nicki 1984 {published data only}
-
- Nicki RM, Remington RE, MacDonald GA. Self‐efficacy, nicotine‐fading/self‐monitoring and cigarette‐smoking behaviour. Behaviour Research and Therapy 1984;22(5):477‐85. - PubMed
Ostroff 2014 {published data only}
-
- NCT00575718. Smoking cessation intervention for cancer patients. clinicaltrials.gov/ct2/show/NCT00575718 (first received 18 December 2007).
-
- Sloan E, Lord‐Bessen J, Hollan S, Li Y, Burkhalter J, Furberg A, et al. An analysis of treatment implementation fidelity as a possible explanation for null findings in a smoking cessation trial. Society for Research on Nicotine and Tobacco 18th Annual Meeting; 2012 Mar 13‐16; Houston, USA. 2012:101. [POS3‐32]
Perez‐Milena 2012 {published data only}
-
- Perez‐Milena A, Navarreteguillen AB, Mesa‐Gallardo MI, Martinez Perez R, Leal‐Helmling FJ, Perez‐Fuentes C. Efficiency of two motivational interventions for adolescent smokers (brief and intensive) conducted in High Schools [Eficiencia de dos intervenciones motivacionales para la deshabituación tabáquica en adolescentes (breve e intensiva) realizadas en Institutos de Educación Secundaria]. Adicciones 2012;24(3):191‐9. - PubMed
Rennard 2006 {published data only}
-
- Rennard SI, Glover E, Leischow S, Daughton DM, Glover P, Muramoto M. Efficacy of nicotine inhaler in smoking reduction. Nicotine & Tobacco Research 2002;4(3):380. - PubMed
-
- Rennard SI, Glover ED, Leischow S, Daughton DM, Glover PN, Muramoto M, et al. Efficacy of the nicotine inhaler in smoking reduction: A double‐blind, randomized trial. Nicotine & Tobacco Research 2006;8(4):555‐64. - PubMed
Riley 2001 {published data only}
-
- Jerome A, Fiero PL, Behar A, Bensur A. Combining scheduled, gradual reduction with nicotine replacement. Society for Research on Nicotine and Tobacco Fifth Annual Meeting; 1999 Mar 5‐7; San Diego, USA. 1999. [PO B50]
-
- Riley W, Behar A, Jerome A, Weil WS. Computerized scheduled gradual reduction of smoking prior to nicotine patch use: evidence of additive efficacy. Society for Research on Nicotine and Tobacco 7th Annual Meeting; 2001 Mar 20‐23 Seattle, USA. 2001:74. [PO3 28]
Riley 2005 {published data only}
-
- Riley W. Computerized scheduling of nicotine nasal spray. USA: LifeSign; 2005. Study ID: R44DA013062.
-
- Riley WT, Behar A, Shields R, Kelleher K, Vasiliadis M. Effects of a scheduled gradual crossover of cigarettes and nicotine nasal spray. Society for Research on Nicotine and Tobacco 11th Annual Meeting; 2005 Mar 20‐23; Prague, Czech Republic. 2005.
Rohsenow 2016 {published data only}
Ruther 2018 {published data only}
-
- Ruther T, Kiss A, Eberhardt K, Linhardt A, Kroger C, Pogarell O. Evaluation of the cognitive behavioral smoking reduction program "Smoke_less": a randomized controlled trial. European Archives of Psychiatry and Clinical Neuroscience 2018;268(3):269‐77. - PubMed
Shiffman 2009 {published data only}
-
- Shiffman S, Dresler CM, Norton M, Strahs K. Using nicotine gum to assist quitting by gradual reduction. Society for Research on Nicotine and Tobacco 12th Annual Meeting; 2006 Feb 15‐18, Orlando, USA. 2006:14. [SYM10D]
-
- Shiffman S, Ferguson SG, Strahs KR. Quitting by gradual smoking reduction using nicotine gum: a randomized controlled trial. American Journal of Preventive Medicine 2009;36(2):96‐104. - PubMed
Wang 2017 {published data only}
-
- Wang MP, Li WH, Cheung YT, Lam OB, Wu Y, Kwong AC, et al. Brief advice on smoking reduction versus abrupt quitting for smoking cessation in Chinese smokers: a cluster randomized controlled trial. Nicotine & Tobacco Research 2017;20(1):67‐72. - PubMed
Wennike 2003 {published data only}
-
- Wennike P, Danielsson T, Landfeldt B, Westin A, Tonnesen P. Smoking reduction promotes smoking cessation: results from a double blind, randomized, placebo‐controlled trial of nicotine gum with 2‐year follow‐up. Addiction 2003;98(10):1395‐402. - PubMed
Wu 2017 {published data only}
-
- Wu L, He Y, Jiang B, Zhang D, Tian H, Zuo F, et al. Very brief physician advice and supplemental proactive telephone calls to promote smoking reduction and cessation in Chinese male smokers with no intention to quit: a randomized trial. Addiction 2017;112(11):2032‐40. - PubMed
References to studies excluded from this review
ACTRN12609000482268 {published data only}
-
- ACTRN12609000482268. Randomised controlled trial of Zonnic and nicotine patch (ZAP) in smoking cessation [Randomised placebo‐controlled trial of Zonnic and nicotine patch in heavy smokers for smoking cessation]. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12609000482268 (First received 18 June 2009).
ACTRN12617000905369 {published data only}
-
- ACTRN12617000905369. Feasibility of electronic nicotine devices for smoking cessation with alcohol and other drug treatment clients [The QuitENDs Study: a feasibility pilot study of electronic nicotine devices for smoking cessation with alcohol and other drug treatment clients]. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=373092 (First received 20 June 2017).
Applegate 2004 {published data only}
-
- Applegate BW, Riley WT, Sowell A. A comparison of computer‐assisted scheduled gradual reduction vs. self help in unmotivated smokers. Society for Research on Nicotine and Tobacco 10th Annual Meeting; 2004 Feb 18‐21; Phoenix, USA. 2004:41. [POS1‐028]
Armitage 2007 {published data only}
Atwood 1975 {published data only}
-
- Atwood JT. The Influence of Differential Treatment Goals on the Success of a Behavior Modification Program for Smokers [thesis]. USA: Dissertation Abstracts International, 1975.
Audrain McGovern 2011 {published data only}
Aveyard 2011 {published data only}
-
- Aveyard P, Begh R, Sheikh A, Amos A. Promoting smoking cessation through smoking reduction during Ramadan. Addiction 2011;106(8):1379‐80. [DOI: ] - PubMed
Aveyard 2014 {published data only}
-
- Aveyard P, Lindson‐Hawley N, Hastings G, Andrade M. Should smokers be advised to cut down as well as quit?. BMJ 2014;348:g2787. [DOI: ] - PubMed
Baker 2006 {published data only}
-
- Baker A, Richmond R, Kay‐Lambkin F, Lewin TJ, Carr VJ. Randomized controlled trial of a smoking cessation intervention among people with a psychotic disorder: 3‐year follow‐up. Society for Research on Nicotine and Tobacco 13th Annual Meeting; 2007 Feb 21‐24, Austin, USA. 2007. [PA2‐2]
-
- Lan TH, Chiu HJ, Wu BJ, Hung TH, Hu TM. Readiness to quit and smoking reduction outcomes. American Journal of Psychiatry 2007;164(5):827‐8. - PubMed
Barbarin 1978 {published data only}
-
- Barbarin OA. Comparison of symbolic and overt aversion in the self‐control of smoking. Journal of Consulting and Clinical Psychology 1978;46(6):1569‐71. - PubMed
Batra 2003 {published data only}
-
- Batra A, Haustein K‐O, Friederich HM, Zschatsch I. Controlled smoking reduction ‐ results of a placebo‐controlled smoking reduction study with nicotine chewing gum [Kontrollierte rauchreduktion ‐ ergebnisse aus einer placebo‐kontrollierten rauchreduktionsstudie mit Nikotinkaugummi]. Verhaltenstherapie 2003;13(S1):5.
Batra 2005 {published data only}
-
- Landfeldt B, Batra A, Friederich HM, Klingler K, Westin A. Smoking reduction with a 4 mg nicotine gum ‐ final results from a placebo‐controlled trial over 13 months. Society for Research on Nicotine and Tobacco 5th European Meeting; 2003 Nov 20‐22; Padua, Italy. 2003.
Beavers 1973 {published data only}
-
- Beavers ME. Smoking Control: a Comparison of Three Aversive Conditioning Treatments [thesis]. USA: Dissertation Abstracts International, 1973.
Becona 1991 {published data only}
-
- Becona E, Gomez‐Duran BJ. Reduction of cigarette intake at baseline and efficacy of a smoking cessation programme [Descenso del consumo de cigarrillos en la linea base y eficacia de un programa para dejar de fumar]. Revista Española de Drogodependencias 1991;16(4):277‐84.
Becona 1993 {published data only}
-
- Becona E, Garcia MP. Nicotine fading and smokeholding methods to smoking cessation. Psychological Reports 1993;73(3):779‐86. - PubMed
Becona 1998 {published data only}
-
- Becona E, Vazquez FL. Efficacy of a behavioral multicomponent cessation program at five year follow‐up. Research Communications in Biological Psychology & Psychiatry 1998;23(1‐2):9‐17.
Becona 2001 {published data only}
-
- Becona E, Vazquez FL. Comparison of the efficacy of a self‐help smoking cessation by mail with a manual in one‐time intervention or weekly intervention [Comparacion de la eficacia de un programa de autoayuda para dejar de fumar distriuido por correo en un manual en un unico envio o semanalmente en folletos]. Psiquis 2001;22(4):41‐50.
Becona Iglesias 1989 {published data only}
-
- Becona Iglesias E, Lista Rubianes MJ. Treatment of smokers with nicotine fading techniques. Psiquis Revista de Psiquiatria, Psicologia y Psicosomatica 1989;10(4):38‐44.
Berecz 1971 {published data only}
-
- Berecz JM. Reduction of smoking through self‐administered aversion conditioning of imagined behavior. Annual Convention of the American Psychological Association. 1971:429‐30.
Berecz 1984 {published data only}
-
- Berecz, JM. Superiority of a low‐contrast smoking cessation method. Addictive Behaviors 1984;9(3):273‐8. [DOI: ] - PubMed
Berger 2006 {published data only}
-
- Berger, J. Smoking reduction and lung cancer risk. JAMA 2006;295(9):1001‐2. - PubMed
Bernard 1972 {published data only}
-
- Bernard HS, Efran JS. Eliminating versus reducing smoking using pocket timers. Behaviour Research and Therapy 1972;10(4):399‐401. - PubMed
Blalock 2001 {published data only}
-
- Blalock JA, Cinciripini PM, Hogan MC. Transdermal nicotine and gradual reduction for smoking cessation. Society for Research on Nicotine and Tobacco 7th Annual Meeting, 2001 Mar 21‐23; Seattle, USA. 2001:74. [PO3 31]
Bloch 2010 {published data only}
-
- Bloch B, Reshef A, Cohen T, Tafla A, Gathas S, Israel S, et al. Preliminary effects of bupropion and the promoter region (HTTLPR) serotonin transporter (SLC6A4) polymorphism on smoking behavior in schizophrenia. Psychiatry Research 2010;175(1‐2):38‐42. [DOI: 10.1016/j.psychres.2008.12.015] - DOI - PubMed
Bolliger 2000b {published data only}
-
- Bolliger CT. Practical experiences in smoking reduction and cessation. Addiction 2000;95(S1):19‐24. - PubMed
Borland 1999 {published data only}
Borland 2013 {published data only}
Bowers 1987 {published data only}
-
- Bowers TG, Winett RA, Frederiksen LW. Nicotine fading, behavioral contracting, and extended treatment: effects on smoking cessation. Addictive Behaviors 1987;12(2):181‐4. - PubMed
Bradford 1991 {published data only}
-
- Bradford TS. The Effects of Combining Behavioral Counselling with Nicotine Fading and Smoke Holding in Medically At‐Risk Adult Smokers [thesis]. USA: Dissertation Abstracts International, 1991.
Brown 1984 {published data only}
-
- Brown RA, Lichtenstein E, McIntyre KO, Harrington‐Kostur J. Effects of nicotine fading and relapse prevention on smoking cessation. Journal of Consulting and Clinical Psychology 1984;52(2):307‐8. [DOI: ] - PubMed
Brue 2001 {published data only}
-
- Brue V, Karlson K, Escamilla G. Utility of a computerized device in scheduled reduced smoking. Society for Research on Nicotine and Tobacco 7th Annual Meeting; 2001 Mar 21‐23; Seattle, USA. 2001:106. [PO2 78)]
Buceta 1989 {published data only}
-
- Buceta JM, Lopez A. Application of a behavioral‐cognitive intervention program for smoking cessation. Revista Intercontinental de Psicologia y Educacion 1989;2(3):81‐99.
Burling 1982 {published data only}
-
- Burling TA, Stitzer ML, Bigelow GE, Russ NW. Techniques used by smokers during contingency motivated smoking reduction. Addictive Behaviors 1982;7(4):397‐401. - PubMed
Burling 1989 {published data only}
-
- Burling TA, Marotta J, Gonzalez R, Moltzen JO, Eng AM, Schmidt GA, et al. Computerized smoking cessation program for the worksite: treatment outcome and feasibility. Journal of Consulting and Clinical Psychology 1989;57(5):619‐22. - PubMed
Burling 1994 {published data only}
-
- Burling TA, Seidner AL, Gaither DE. A computer‐directed program for smoking cessation treatment. Journal of Substance Abuse 1994;6(4):427‐31. - PubMed
Burris 2014 {published data only}
Cacciapaglia 2007 {published data only}
-
- Cacciapaglia HM, Kaplar ME, McConnell K, Schwetschenau HM, Carels RA. A stepped‐care approach to smoking cessation and harm reduction. Annals of Behavioral Medicine 2007;33(S1):205.
Caponnetto 2013 {published data only}
-
- Caponnetto P, Campagna D, Cibella F, Morjaria JB, Caruso M, Russo C, et al. Efficiency and safety of an electronic cigarette (ECLAT) as tobacco cigarettes substitute: a prospective 12‐month randomized control design study. PloS One 2013;8(6):e66317. [DOI: 10.1371/journal.pone.0066317] - DOI - PMC - PubMed
-
- NCT01194583. Randomised controlled trial investigating the efficacy and safety of an electronic nicotine delivery device (e‐cigarette) without nicotine cartridges in smokers. clinicaltrials.gov/show/nct01194583 (first received 3 September 2010).
Caponnetto 2014 {published data only}
Cassidy 2018 {published data only}
-
- Cassidy RN, Jackson KM, Rohsenow DJ, Tidey JW, Tevyaw TO, Barnett NP, et al. Contingency management for college student smokers: the role of drinking as a moderator and mediator of smoking abstinence during treatment. Addictive Behaviors 2018;80:95‐101. [DOI: 10.1016/j.addbeh.2018.01.017] - DOI - PMC - PubMed
Chambliss 1979 {published data only}
-
- Chambliss C, Murray EJ. Cognitive procedures for smoking reduction: symptom attribution versus efficacy attribution. Cognitive Therapy and Research 1979;3(1):91‐5.
Chen 2013 {published data only}
-
- Chen HK, Lan TH, Wu BJ. A double‐blind randomized clinical trial of different doses of transdermal nicotine patch for smoking reduction and cessation in long‐term hospitalized schizophrenic patients. European Archives of Psychiatry and Clinical Neuroscience 2012;263(1):75‐82. [DOI: 10.1007/s00406-012-0338-3] - DOI - PubMed
Cinciripini 1994 {published data only}
-
- Cinciripini PM, Lapitsky LG, Wallfisch A, Mace R, Nezami E, Vunakis H. An evaluation of a multicomponent treatment program involving scheduled smoking and relapse prevention procedures: initial findings. Addictive Behaviors 1994;19(1):13‐22. - PubMed
Colby 2005 {published data only}
-
- Colby SM, Monti PM, O'Leary Tevyaw T, Barnett NP, Spirito A, Rohsenow DJ, et al. Brief motivational intervention for adolescent smokers in medical settings. Addictive Behaviors 2005;30(5):865‐74. - PubMed
Colletti 1978 {published data only}
-
- Colletti G. The Relative Efficacy of Participant Modeling, Participant Observer, and Self‐monitoring Procedures as Maintenance Strategies Following a Positive Behaviorally Based Treatment for Smoking Reduction [thesis]. USA: Dissertation Abstracts International, 1978.
Colletti 1979 {published data only}
-
- Colletti G, Stern L. Two‐year follow‐up of a nonaversive treatment for cigarette smoking. Journal of Consulting and Clinical Psychology 1980;48(2):292‐3. - PubMed
Colletti 1980 {published data only}
-
- Colletti G, Supnick JA. Continued therapist contact as a maintenance strategy for smoking reduction.. Journal of Consulting and Clinical Psychology 1980;48(5):665‐7. [DOI: ] - PubMed
Colletti 1982 {published data only}
-
- Colletti G, Supnick JA, Rizzo AA. Long‐term follow‐up (3‐4 years) of treatment for smoking reduction. Addictive Behaviors 1982;7(4):429‐33. - PubMed
Corty 1984 {published data only}
-
- Corty E, McFall RM. Response prevention in the treatment of cigarette smoking.. Addictive Behaviors 1984;9(4):405‐8. - PubMed
Cropsey 2008 {published data only}
Cropsey 2015 {published data only}
-
- Cropsey KL, Clark CB, Hendricks PS. Varenicline for smoking reduction prior to cessation. JAMA 2015;313(22):2284‐5. [DOI: ] - PubMed
Crosbie 1972 {published data only}
-
- Crosbie PV, Petroni FA, Stitt BG. The dynamics of "corrective" groups. Journal of Health and Social Behavior 1972;13(3):294‐302. [DOI: ] - PubMed
D'Ruiz 2017 {published data only}
-
- D'Ruiz CD, O'Connell G, Graff DW, Yan XS. Measurement of cardiovascular and pulmonary function endpoints and other physiological effects following partial or complete substitution of cigarettes with electronic cigarettes in adult smokers. Regulatory Toxicology and Pharmacology 2017;87:36‐53. [DOI: 10.1016/j.yrtph.2017.05.002] - DOI - PubMed
Darity 1997 {published data only}
-
- Darity WA, Chen TT, Tuthill RW, Buchanan DR, Winder AE, Stanek E, et al. A multi‐city community based smoking research intervention project in the African‐American population. International Quarterly of Community Health Education 1997;17(2):117‐30. [DOI: ] - PubMed
Daughton 1994 {published data only}
-
- Daughton DM, Millatmal T, Susman JL, Belenky S, Sitorius M, Floreani AA, et al. Abrupt abstinence is critical to smoking cessation success in a family practice setting. Journal of Smoking‐Related Disorders 1994;5(S1):319.
Delahunt 1977 {published data only}
-
- Delahunt JG. Effectiveness Assessment of Modified Treatments for the Elimination of Smoking [thesis]. USA: Dissertation Abstracts International, 1977.
Dlack 1999 {published data only}
-
- Dlack GW, Meador‐Woodruff JH. Nicotine replacement and smoking reduction in smokers with schizophrenia. Schizophrenia Research 1999;1‐3:276. - PubMed
Dogris 1998 {published data only}
-
- Dogris NJ. The Effect of a Prerecorded Hypnosis CD and Behavioral Fading on Smoking Cessation and Locus of Control [thesis]. USA: Dissertation Abstracts International, 1998.
Ebbert 2010 {published data only}
Ehrsam 1991 {published data only}
-
- Ehrsam RE, Buhler A, Muller P, Mauli D, Schumacher PM, Howald H, et al. Weaning of young smokers using a transdermal nicotine patch. Schweizerische Rundschau fur Medizin Praxis (Revue Suisse de Medecine Praxis) 1991;80(7):145‐50. - PubMed
Elliott 1978 {published data only}
-
- Elliott CH, Denney DR. A multiple component treatment approach to smoking reduction. Journal of Consulting and Clinical Psychology 1978;46(6):1330‐9. [DOI: ] - PubMed
Emmons 1988 {published data only}
-
- Emmons KM, Emont SL, Collins RL, Weidner G. Relapse prevention versus broad spectrum treatment for smoking cessation: a comparison of efficacy. Journal of Substance Abuse 1988;1(1):79‐89. - PubMed
Etringer 1984 {published data only}
-
- Etringer BD, Gregory VR, Lando HA. Influence of group cohesion on the behavioral treatment of smoking. Journal of Consulting and Clinical Psychology 1984;52(6):1080‐6. [DOI: ] - PubMed
Etter 2011 {published data only}
Euler 1973 {published data only}
-
- Euler VHA. Reduction of cigarette smoking through self‐monitoring [Die reduktion des zigarettenrauchens durch selbst‐monitoring]. Zeitschrift fur Klinische Psychologie und Psychotherapie 1973;21(3):271‐82. - PubMed
Evins 2001 {published data only}
-
- Evins AE, Cather C, Rigotti NA, Freudenreich O, Henderson DC, Olm Shipman CM, et al. Two‐year follow‐up of a smoking cessation trial in patients with schizophrenia: increased rates of smoking cessation and reduction. Journal of Clinical Psychiatry 2004;65(3):307‐11. - PubMed
-
- Evins AE, Mays VK, Rigotti NA, Tisdale T, Daigle A, Goff DC. Reduction In tobacco use in schizophrenia with bupropion SR and Cognitive Behavioral Therapy. Society for Research on Nicotine and Tobacco 6th Annual Meeting; 2000 Feb 18‐20; Arlington, USA. 2000.
Evins 2007 {published data only}
-
- Evins AE, Cather C, Culhane M, Birnbaum AS, Horowitz J, Hsieh E, et al. A placebo‐controlled study of bupropion SR added to high dose nicotine replacement therapy for smoking cessation or reduction in schizophrenia. Society for Research on Nicotine and Tobacco 12th Annual Meeting; 2006 Feb 15‐18; Orlando, USA. 2006:101. [POS2‐104]
-
- Evins AE, Cather C, Culhane MA, Birnbaum A, Horowitz J, Hsieh E, et al. A 12‐week double‐blind, placebo‐controlled study of bupropion SR added to high‐dose dual nicotine replacement therapy for smoking cessation or reduction in schizophrenia. Journal of Clinical Psychopharmacology 2007;27(4):380‐6. [DOI: 10.1097/01.jcp.0b013e3180ca86fa] - DOI - PubMed
-
- NCT00307203. Safety and effectiveness of sustained release bupropion in treating individuals with schizophrenia who smoke. clinicaltrials.gov/show/NCT00307203 (first received 27 March 2006).
Fagerström 2002a {published data only}
-
- Fagerström K. Smoking reduction in the management of COPD. Monaldi Archives for Chest Disease (Archivio Monaldi per le malattie del torace) 2002;57(5‐6):281‐4. - PubMed
Farkas 2001 {published data only}
-
- Farkas AJ, Pierce JP, Zhu SH. Home smoking restrictions: a brief cessation intervention for callers to the California Smoker's Helpline who are not ready to quit. Society for Research on Nicotine and Tobacco 7th Annual Meeting; 2001 March 21‐23; Seattle, USA. 2001:109. [PO4 48]
Fatemi 2005 {published data only}
Fatemi 2013 {published data only}
-
- Fatemi SH, Yousefi MK, Kneeland RE, Liesch SB, Folsom TD, Thuras PD. Antismoking and potential antipsychotic effects of varenicline in subjects with schizophrenia or schizoaffective disorder: a double‐blind placebo and bupropion‐controlled study. Schizophrenia Research 2013;146(1‐3):376‐8. [DOI: ] - PubMed
Feryo 2009 {published data only}
-
- Feryo MR. Self‐Directed Smoking Cessation Program [thesis]. USA: Dissertation Abstracts International, 2009.
Filia 2010 {published data only}
-
- Filia S, Baker A, Richmond R, Kay‐Lambkin F, Castle D, Williams J, et al. Randomised controlled trial of a healthy lifestyles intervention to reduce cardiovascular disease (CVD) risk among smokers with psychosis: interim results. Australian and New Zealand Journal of Psychiatry 2010;44(S1):A31‐2. [DOI: ]
Forgays 1987 {published data only}
-
- Forgays DG. Flotation rest as a smoking intervention. Addictive Behaviors 1987;12(1):85‐90. [DOI: ] - PubMed
Foxx 1979 {published data only}
-
- Foxx RM, Brown RM, Katz I. Nicotine fading and self‐monitoring for cigarette abstinence or controlled smoking: a two and one‐half year follow‐up. Behavior Therapist 1981;4(2):21‐3.
Foxx 1983 {published data only}
-
- Foxx RM, Axelroth E. Nicotine fading, self‐monitoring and cigarette fading to produce cigarette abstinence or controlled smoking. Behaviour Research and Therapy 1983;21(1):17‐27. [DOI: ] - PubMed
Franklin 2009 {published data only}
Frederiksen 1976 {published data only}
-
- Frederiksen LW, Peterson Gl. Controlled smoking: development and maintenance. Addictive Behaviors 1976;1:193‐6.
Fuhrer 1972 {published data only}
-
- Fuhrer RE. The Effects of Covert Sensitization with Relaxation Induction, Covert Sensitization Without Relaxation Induction, and Attention‐Placebo on the Reduction of Cigarette Smoking [thesis]. USA: Dissertation Abstracts International, 1972.
Galizia 1990 {published data only}
-
- Galizia VJ. A Test of a Compliance Model for Prediction of Smoking Reduction in Cardiopulmonary Patients [thesis]. USA: Dissertation Abstracts International, 1990.
Gariti 2002 {published data only}
-
- Gariti P, Alterman A, Mulvaney F, Mechanic K, Dhopesh V, Yu E, et al. Nicotine intervention during detoxification and treatment for other substance use. American Journal of Drug and Alcohol Abuse 2002;28(4):671‐9. - PubMed
Gelkopf 2012 {published data only}
Gil Roales‐Nieto 1992b {published data only}
-
- Gil Roales‐Nieto J. Nicotine fading and external control fading as a procedure for controlling smoking. Psicothema 1992;4(2):397‐412.
Glasgow 1983 {published data only}
-
- Glasgow RE, Klesges RC, Godding PR, Gegelman R. Controlled smoking, with or without carbon monoxide feedback, as an alternative for chronic smokers. Behavior Therapy 1983;14(3):386‐97.
Glasgow 1984 {published data only}
-
- Glasgow RE, Klesges RC, Godding PR, Vasey MW, O'Neill HK. Evaluation of a worksite‐controlled smoking program. Journal of Consulting and Clinical Psychology 1984;52(1):137‐8. - PubMed
-
- Glasgow RE, Klesges RC, Klesges LM. Long‐term effects of a controlled smoking program: a 2 1/2 year follow‐up. Behavior Therapy 1985;16(3):303. [DOI: ]
Glasgow 1986 {published data only}
-
- Glasgow RE, Klesges RC, O'Neill HK. Programming social support for smoking modification: an extension and replication. Addictive Behaviors 1986;11(4):453‐7. - PubMed
Glasgow 2009b {published data only}
Gonzalez 1991 {published data only}
-
- Gonzalez RC. Cognitive Assessment in Computerized Nicotine Fading [thesis]. USA: Dissertation Abstracts International, 1991.
Gradl 2009 {published data only}
-
- Gradl S, Kroeger C, Floeter S, Piontek D. Effectiveness of a modern smoking cessation programme based on international guidelines. Verhaltenstherapie und Verhaltensmedizin 2009;30(2):169‐85.
Graff 1966 {published data only}
-
- Graff H, Hammet VO, Bash N, Fackler G, Yanovski A, Goldman A. Results of four antismoking methods. Pennsylvania Medical Journal 1966;69:86‐9. - PubMed
Gulliver 2008 {published data only}
-
- Gulliver SB, Kamholz BW. Helstrom AW, Morissette SB, Kahler CW. A preliminary evaluation of adjuncts to motivational interviewing for psychiatrically complex smokers. Journal of Dual Diagnosis 2008;4(4):394‐413. [DOI: 10.1080/15504260802086149] - DOI
Gutmann 1967 {published data only}
-
- Gutmann M, Marston A. Problems of S's motivation in a behavioral program for reduction of cigarette smoking. Psychological Reports 1967;20(3 (2)):1107‐14. [DOI: ]
Gylys 2000 {published data only}
-
- Gylys JA. Smoking Cessation in a Public Health Primary Care Clinic: A Minimal Assistance Scheduled Smoking Approach [thesis]. USA: Dissertation Abstracts International:, 2000.
Hamilton 1998 {published data only}
-
- Hamilton BD. Nicotine Fading Versus Abrupt Quitting: Testing Educational and Behavioral Smoking Cessation Treatment Strategies [thesis]. USA: Dissertation Abstracts International, 1998.
Hatsukami 1988 {published data only}
-
- Hatsukami DK, Dahlgren L, Zimmerman R, Hughes JR. Symptoms of tobacco withdrawal from total cigarette cessation versus partial cigarette reduction. Psychopharmacology 1988;94(2):242‐7. - PubMed
Hatsukami 2005 {published data only}
-
- Kotlyar M, Jensen J, Li Shelby, Hatsukami DK. Effect of smoking reduction on cardiovascular biomarkers and subjective measures. Nicotine & Tobacco Research 2004;6(4):719.
Hawk 2015 {published data only}
Hilleman 1994 {published data only}
-
- Hilleman DE, Mohiuddin SM, Delcore MG. Comparison of fixed‐dose transdermal nicotine, tapered‐dose transdermal nicotine, and buspirone in smoking cessation. Journal of Clinical Pharmacology 1994;34(3):222‐4. - PubMed
Hills 1982 {published data only}
-
- Hills AA. Target‐setting self‐control for smoking. Psychological Reports 1982;50(1):68‐70. [DOI: ] - PubMed
Hovell 2009 {published data only}
Hughes 1991 {published data only}
-
- Hughes JR, Gust SW, Keenan R, Fenwick JW, Skoog K, Higgins ST. Long‐term use of nicotine vs placebo gum. Archives of Internal Medicine 1991;151(10):1993‐8. - PubMed
Hughes 2004b {published data only}
Hughes 2007 {published data only}
-
- Hughes JR. Smokers who choose to quit gradually versus abruptly. Addiction 2007;102(8):1326‐7. - PubMed
Hughes 2011 {published data only}
-
- Hughes JR, Rennard SI, Fingar JR, Talbot SK, Callas PW, Fagerström KO. Efficacy of varenicline to prompt quit attempts in smokers not currently trying to quit: a randomized placebo‐controlled trial. Nicotine & Tobacco Research 2011;13(10):955‐64. [10.1093/ntr/ntr103 MISC1 ‐ DC:20110928] - PMC - PubMed
Hughes 2016 {published data only}
-
- Hughes JR, Klemperer EM. Gradual versus abrupt smoking cessation. Annals of Internal Medicine 2016;165(10):741. [DOI: ] - PubMed
Hurt 1990 {published data only}
-
- Hurt RD, Lauger GG, Offord KP, Kottke TE, Dale LC. Nicotine‐replacement therapy with use of a transdermal nicotine patch ‐ a randomized double‐blind placebo‐controlled trial. Mayo Clinic Proceedings 1990;65(12):1529‐37. - PubMed
ISRCTN13288677 {published data only}
-
- ISRCTN13288677. Can electronic cigarettes and nicotine replacement treatment help reduce smoking in smokers who struggle to quit?. www.isrctn.com/ISRCTN13288677 (first received 17 March 2017).
ISRCTN13837944 {published data only}
-
- ISRCTN13837944. Improving behavioural support for reducing smoking among those who want to cut down [An exploratory trial to evaluate the effects of a physical activity intervention as a smoking cessation induction and cessation aid among the 'hard to reach']. www.isrctn.com/ISRCTN13837944 (first received 5 July 2010).
ISRCTN64013828 {published data only}
-
- ISRCTN64013828. The non‐concealed placebo: a randomized trial on smoking cessation [An open‐label randomized interventional study to assess the effect of placebo smoking cessation patches versus no treatment on nicotine dependence scores of adult Filipino smokers]. www.isrctn.com/ISRCTN64013828 (first received 24 February 2016).
Jacobs 1971 {published data only}
-
- Jacobs MA, Spilken AZ, Norman MM, Wohlberg GW, Knapp PH. Interaction of personality and treatment conditions associated with success in a smoking control program. Psychosomatic Medicine 1971;33(6):545‐56. - PubMed
Jeon 2016 {published data only}
Jerome 1999b {published data only}
-
- Jerome A, Fiero PL. Computerized scheduling of nicotine gum use (personal communication. email to: Lindson N 1999.
Joksic 2011 {published data only}
Joseph 2005 {published data only}
-
- Joseph A, Hecht S, Murphy S, Gross M, Lando H, Bliss R, et al. A randomized controlled trial of smoking reduction in heart disease patients. Nicotine & Tobacco Research 2005;7(4):687.
Karam‐Hage 2014 {published data only}
Karoly 1975 {published data only}
-
- Karoly P, Doyle WW. Effects of outcome expectancy and timing of self monitoring on cigarette smoking. Journal of Clinical Psychology 1975;31(2):351‐5. - PubMed
KCT0001277 {published data only}
-
- KCT0001277. Effect of an electronic cigarette for smoking reduction and cessation in Korean male smokers: a randomized, controlled study. apps.who.int/trialsearch/Trial3.aspx?trialid=KCT0001277 (first received 14 November 2014).
Kelly 2010 {published data only}
-
- Kelly AC, Zuroff DC, Foa CL, Gilbert P. Who benefits from training in self‐compassionate self‐regulation? A study of smoking reduction. Journal of Social and Clinical Psychology 2010;29(7):727‐55. [DOI: ]
Keutzer 1968 {published data only}
-
- Keutzer CS. Behavior modification of smoking: the experimental investigation of diverse techniques. Behaviour Research and Therapy 1968;6(2):137‐57. [DOI: ] - PubMed
Klein 2010 {published data only}
-
- Klein G, Ulbricht S, Haug S, Gross B, Rumpf H‐J, John U, et al. Effects of practitioner‐delivered brief counseling and computer‐generated tailored letters on cigarettes per day among smokers who do not quit ‐ a quasi‐randomized controlled trial. Drug and Alcohol Dependence 2010;112(1‐2):81‐9. [DOI: ] - PubMed
Klemperer 2016 {published data only}
-
- Klemperer EM, Hughes JR. After precessation nicotine replacement therapy, abrupt cessation increases abstinence more than gradual cessation in smokers ready to quit. Evidence‐Based Medicine 2016;21(5):174. [DOI: ] - PubMed
Lamb 2004 {published data only}
Lamb 2007 {published data only}
Lamb 2010 {published data only}
Lan 2007 {published data only}
-
- Lan TH, Chiu HJ, Wu BJ, Hung TH, Hu TM. Readiness to quit and smoking reduction outcomes.. American Journal of Psychiatry 2007;164(5):827‐8. - PubMed
Lando 1985 {published data only}
-
- Lando HA, McGovern PG. Nicotine fading as a nonaversive alternative in a broad‐spectrum treatment for eliminating smoking. Addictive Behaviors 1985;10(2):153‐61. - PubMed
Larson 1999 {published data only}
-
- Larson ME. Behavioral Substitutes and Smoking Cessation: Do They Make Quitting Easier? [thesis]. USA: Dissertation Abstracts International, 1999.
Leischow 2004 {published data only}
-
- Leischow SJ, Djordjevic MV. Smoking reduction and tobacco‐related cancers: the more things change, the more they stay the same. Journal of the National Cancer Institute 2004;96(2):86‐7. - PubMed
Levinson 1971 {published data only}
-
- Levinson BL, Shapiro D, Schwartz GE, Tursky B. Smoking elimination by gradual reduction. Behavior Therapy 1971;2(4):477‐87. [DOI: ]
Levinson 2008 {published data only}
-
- Levinson AH, Glasgow RE, Gaglio B, Smith TL, Cahoon J, Marcus AC. Tailored behavioral support for smoking reduction: development and pilot results of an innovative intervention. Health Education Research 2008;23(2):335‐46. - PubMed
Lichtenstein 1967 {published data only}
-
- Lichtenstein E, Poussaint AF, Bergman SH, Jurney T, Shapiro R. A further report on the effects of the physician's treating of smoking by placebo. Diseases of the Nervous System 1967;28(11):754‐5. - PubMed
Lillington 1998 {published data only}
-
- Lillington GA, Sachs DP. Preoperative smoking reduction: all or nothing at all?. Chest 1998;113(4):856‐8. - PubMed
Macgregor 1996 {published data only}
-
- Macgregor ID. Efficacy of dental health advice as an aid to reducing cigarette smoking. British Dental Journal 1996;180(8):292‐6. - PubMed
Marston 1971 {published data only}
-
- Marston AR, McFall RM. Comparison of behavior modification approaches to smoking reduction. Journal of Consulting and Clinical Psychology 1971;36(2):153‐62. [DOI: ] - PubMed
Meredith 2011 {published data only}
Mihaltan 2007 {published data only}
-
- Mihaltan F. Smoking reduction ‐ an alternative solution? [Reducerea fumatului ‐ o solutie alternativa pentru alte oferte?]. Pneumologia 2007;56(4):218‐20. - PubMed
Morris 2011 {published data only}
Mueller 2012 {published data only}
-
- Mueller S, Petitjean S, Degen B, Wiesbeck GA. Smoking cessation in alcohol‐dependent patients: Results from a randomized, controlled trial. Alcohol and Alcoholism 2011;46:i7.
Muramoto 1999 {published data only}
-
- Muramoto ML, Leischow S, Cook G, Castellini SM, Strayer L. Effectiveness of over‐the‐counter transdermal nicotine for smoking reduction. Nicotine & Tobacco Research 1999;1(2):107‐7.
Murray 1981 {published data only}
-
- Murray RG, Hobbs SA. Effects of self‐reinforcement and self‐punishment in smoking reduction: implications for broad‐spectrum behavioral approaches. Addictive Behaviors 1981;6(1):63‐7. - PubMed
Myette 1993 {published data only}
Myles 1994 {published data only}
-
- Myles PS. Smoking reduction and tape suggestion. Anaesthesia 1994;49(10):917‐8. - PubMed
Møller 2002 {published data only}
NCT01772641 {published data only}
-
- NCT01772641. A smoking intervention study using scheduled gradual reduction with varenicline to help with cessation. clinicaltrials.gov/show/nct01772641 (first received 21 January 2013).
NCT01982110 {published data only}
-
- NCT01982110. A mindfulness based application for smoking cessation. clinicaltrials.gov/show/nct01982110 (first received 13 November 2013).
NCT03128554 {published data only}
-
- NCT03128554. Smoking reduction: testing a novel approach to cessation in primary care practice settings. clinicaltrials.gov/show/NCT03128554 (first received 25 April 2017).
Nentwig 1978 {published data only}
-
- Nentwig CG. Attribution of cause and long‐term effects of the modification of smoking behavior. Behavior Analysis and Modification 1978;2(4):285‐95.
Newman 1982 {published data only}
-
- Newman A, Bloom R. Smoking reduction: a comparison of the effectiveness of rapid smoking and increasing delay training. Addictive Behaviors 1982;7(1):93‐6. - PubMed
Noonan 2018 {published data only}
NTR5113 {published data only}
-
- NTR5113. Blended smoking cessation treatment ‐ evaluation of effectiveness of a blended face‐to‐face and web‐based smoking cessation treatment versus treatment as usual (randomised controlled trial) ‐ (LiveSmokefree‐Study). www.trialregister.nl/trial/4975 (first received 24 March 2015).
O'Brien 2015 {published data only}
O'Connor 1998 {published data only}
-
- O'Connor K, Langlois R. Changes in confidence and craving during smoking reduction. Clinical Psychology & Psychotherapy 1998;5:145‐54.
Olbrich 2008 {published data only}
-
- Olbrich R, Trager S, Nikitopoulos J, Mann K, Diehl A. Smoking reduction during inpatient alcohol detoxification: a controlled clinical pilot trial [Rauchreduktion bei alkoholkranken im rahmen einer stationaren alkohol‐entzugsbehandlung: eine kontrollierte klinische pilotstudie]. Fortschritte der Neurologie‐Psychiatrie 2008;76(5):272‐7. [DOI: ] - PubMed
Orleans 1991 {published data only}
-
- Orleans CT, Schoenbach VJ, Wagner EH, Quade D, Salmon MA, Pearson DC, et al. Self‐help quit smoking interventions: effects of self‐help materials, social support instructions, and telephone counseling. Journal of Consulting and Clinical Psychology 1991;59(3):439‐48. - PubMed
Patten 1996 {published data only}
-
- Patten CA. Effectiveness of Cognitive‐Behavioral Depression Therapy as an Adjunct to Smoking Cessation Treatment for Recovering Alcoholics [thesis]. USA: Dissertation Abstracts International, 1996.
Pisinger 2005 {published data only}
-
- Pisinger C. Smoking cessation and smoking reduction in a general population. The Inter99 study. A randomised population‐based intervention study. Nordic Journal of Psychiatry 2006;60(1):67.
Relinger 1977 {published data only}
-
- Relinger H, Bornstein PH, Bugge ID, Carmody TP, Zohn CJ. Utilization of adverse rapid smoking in groups: efficacy of treatment and maintenance procedures. Journal of Consulting and Clinical Psychology 1977;45(2):245‐9. [DOI: ] - PubMed
Rennard 1993 {published data only}
-
- Rennard SI, Daughton DM. Smoking reduction and biological markers of response. Monaldi Archives for Chest Disease (Archivio Monaldi per le Malattie del Torace) 1993;48(5):580‐2. - PubMed
Rennard 2010 {published data only}
-
- Rennard S, Hughes JP, Kralikova E, Raupach T, Arteaga C. Randomized, placebo‐controlled trial of varenicline for smoking cessation with flexible quit dates. European Respiratory Society Annual Congress; 2010 Sept 18‐22; Barcelona, Spain. 2010.
Riggs 2001 {published data only}
-
- Riggs RL, Hughes JR, Pillitteri JL. Two behavioral treatments for smoking reduction: a pilot study. Nicotine & Tobacco Research 2001;3(1):71‐6. - PubMed
Riley 2002 {published data only}
-
- Riley W, Jerome A, Behar A, Weil J. Computer and manual self‐help behavioral strategies for smoking reduction: Initial feasibility and one‐year follow‐up. Nicotine and Tobacco Research 2002;4(S2):183‐8. - PubMed
Ritchie 1991 {published data only}
-
- Ritchie PJ. Abstinence Versus Controlled Smoking as Goals in Behavioural and Educational Smoking Treatment Programmes [thesis]. USA: Dissertation Abstracts International, 1991.
Robey 2015 {published data only}
-
- Robey RB, Block CA, O'Rourke DJ. Varenicline for smoking reduction prior to cessation. JAMA 2015;313(22):2285. [DOI: ] - PubMed
Rose 2010 {published data only}
Rovina 2003 {published data only}
-
- Rovina N, Gratziou C, Nikoloutsou I, Athanassa Z, Francis K, Roussos C. Short or prolonged treatment with bupropion HCL in smoking cessation therapy. European Respiratory Journal 2003;22(S45):165.
Royce 1995 {published data only}
Rutter 1990 {published data only}
-
- Rutter S. Cigarette‐smoking reduction in university students. Psychological Reports 1990;66(1):186. - PubMed
Salk 1976 {published data only}
-
- Salk GC. A Comparison of Two Smoking Reduction Treatments Under Conditions Designed to be Interfering or Not Interfering With the Smoking Habit [thesis]. USA: Dissertation Abstracts International, 1976.
Schiller 2012 {published data only}
-
- Hatsukami D, Anderson A, Jensen J. Immediate vs. gradual reduction toward cessation in smokeless tobacco user. Society for Research on Nicotine & Tobacco 17th Annual Meeting; 2011 Feb 16‐19; Toronto, Canada. 2011. [SYM 12A]
Schinke 1978 {published data only}
-
- Schinke SP, Blythe BJ, Doueck HJ. Reducing cigarette smoking: evaluation of a multifaceted interventive program. Behavioral Engineering 1978;4(4):107‐12.
Schleicher 2010 {published data only}
-
- Schleicher HE. Evaluation of a Cognitive‐Behavioral Mood Management Intervention for Depressed College Smokers [thesis]. USA: Dissertation Abstracts International, 2010.
Schuurmans 2016 {published data only}
-
- Schuurmans MM. Gradual versus abrupt smoking cessation. Annals of Internal Medicine 2016;165(10):741‐2. [DOI: ] - PubMed
Schwartz 1967 {published data only}
-
- Schwartz JL, Dubitzky M. Clinical reduction of smoking: a California study. California's Health 1967;24(6):78‐84.
Scott 1986 {published data only}
-
- Scott RR, Prue DM, Denier CA, King AC. Worksite smoking intervention with nursing professionals: long‐term outcome and relapse assessment. Journal of Consulting and Clinical Psychology 1986;54(6):809‐13. [DOI: ] - PubMed
Severson 2000 {published data only}
-
- Severson HH, Akers L, Andrews JA, Lichtenstein E, Jerome A. Evaluating two self‐help interventions for smokeless tobacco cessation. Addictive Behaviors 2000;25(3):465‐70. - PubMed
Sipich 1974 {published data only}
-
- Sipich JF, Russell RK, Tobias LL. A comparison of covert sensitization and "nonspecific" treatment in the modification of smoking behavior. Journal of Behavior Therapy and Experimental Psychiatry 1974;5(2):201‐3. [DOI: ]
Smith 2017 {published data only}
Spanos 1993 {published data only}
-
- Spanos NP, Sims A, Faye B, Mondoux TJ, Gabora NJ. A comparison of hypnotic and nonhypnotic treatments for smoking. Imagination, Cognition and Personality 1992;12(1):23‐43.
Srivastava 2007 {published data only}
-
- Srivastava GN. Seven steps of gradual cessation of smoking ‐ an example from India. Vojnosanitetski Pregled 2007;64(6):405‐8. - PubMed
Stein 2002 {published data only}
Steinberg 2018 {published data only}
Stitzer 1985 {published data only}
-
- Stitzer ML, Bigelow GE. Contingent reinforcement for reduced breath carbon monoxide levels: target‐specific effects on cigarette smoking. Addictive Behaviors 1985;10(4):345‐9. - PubMed
Suedfeld 1974 {published data only}
-
- Suedfeld P, Ikard FF. Use of sensory deprivation in facilitating the reduction of cigarette smoking. Journal of Consulting and Clinical Psychology 1974;42(6):888‐95. - PubMed
Sutherland 1975 {published data only}
-
- Sutherland A, Amit Z, Golden M, Roseberger Z. Comparison of three behavioral techniques in the modification of smoking behavior. Journal of Consulting and Clinical Psychology 1975;43(4):443‐7. - PubMed
Templer 1969 {published data only}
-
- Templer DI. Aversive conditioning through hypnosis to reduce smoking. Association for Advancement of Behavior Therapy Newsletter 1969;4(3):9.
Thompson 2016 {published data only}
-
- Taylor AH, Thompson TP, Greaves CJ, Taylor RS, Green C, Warren FC, et al. A pilot randomised trial to assess the methods and procedures for evaluating the clinical effectiveness and cost‐effectiveness of Exercise Assisted Reduction then Stop (EARS) among disadvantaged smokers. Health Technology Assessment 2014;18(4):1‐324. [DOI: 10.3310/hta18040] - DOI - PMC - PubMed
-
- Thompson TP, Greaves CJ, Ayres R, Aveyard P, Warren FC, Byng R, et al. An exploratory analysis of the smoking and physical activity outcomes from a pilot randomized controlled trial of an exercise assisted reduction to stop smoking Intervention in disadvantaged groups. Nicotine & Tobacco Research 2016;18(3):289‐97. [DOI: 10.1093/ntr/ntv099] - DOI - PubMed
-
- Thompson TP, Greaves CJ, Ayres R, Aveyard P, Warren FC, Byng R, et al. Lessons learned from recruiting socioeconomically disadvantaged smokers into a pilot randomized controlled trial to explore the role of Exercise Assisted Reduction then Stop (EARS) smoking. Trials 2015;16(1):1. [DOI: 10.1186/1745-6215-16-1] - DOI - PMC - PubMed
Thorndike 2006 {published data only}
-
- Thorndike FP, Friedman‐Wheeler DG, Haaga DA. Effect of cognitive behavior therapy on smokers' compensatory coping skills. Addictive Behaviors 2006;31(9):1705‐10. - PubMed
Thuerauf 2007 {published data only}
-
- Thuerauf N, Lunkenheimer J, Lunkenheimer B, Sperling W, Bleich S, Schlabeck M, et al. Memantine fails to facilitate partial cigarette deprivation in smokers‐‐no role of Memantine in the treatment of nicotine dependency?. Journal of Neural Transmission 2007;114(3):351‐7. - PubMed
Tidey 2002 {published data only}
-
- Tidey JW, O'Neill SC, Higgins ST. Contingent monetary reinforcement of smoking reductions, with and without transdermal nicotine, in outpatients with schizophrenia. Experimental and Clinical Psychopharmacology 2002;10(3):241‐7. - PubMed
Tseng 2016 {published data only}
-
- Tseng TY, Ostroff JS, Campo A, Gerard M, Kirchner T, Rotrosen J, Shelley D. A randomized trial comparing the effect of nicotine versus placebo electronic cigarettes on smoking reduction among young adult smokers. Nicotine & Tobacco Research 2016;18(10):1937‐43. [DOI: 10.1093/ntr/ntw017] - DOI - PMC - PubMed
Tønnesen 2005 {published data only}
Weidberg 2018 {published data only}
Wetter 2006 {published data only}
-
- Wetter D, Cinciripini PM. Palmtop computer‐delivered treatments for smoking cessation. Society for Research on Nicotine and Tobacco 12th Annual Meeting; 2006 Feb 15‐18; Orlando, USA. 2006. [SYM4C]
Wewers 2009 {published data only}
White 2011 {published data only}
-
- White TJ. Effectiveness of a Brief Behavioral Smoking Cessation Intervention in a Residential Substance Abuse Treatment Center [thesis]. USA: Dissertation Abstracts International, 2011.
Wiseman 1998 {published data only}
-
- Wiseman EJ. Nicotine replacement therapy and smoking reduction as an interim goal. JAMA 1998;279(3):194‐5. - PubMed
References to studies awaiting assessment
Cinciripini 2001 {published data only}
-
- Cinciripini PM, Blalock JA, Wetter DW, Genac D, Seyed A, Seay S. Scheduled smoking and transdermal nicotine replacement. Society for Research on Nicotine and Tobacco 7th Annual Meeting; 2001 Mar 20‐23 Seattle, USA. 2001:32.
Cooper 1990 {published data only}
-
- Cooper T, Clayton RR. Behaviour modification and nicotine reduction therapy with heavy smokers: Comparison of four different dosing strategies. 7th World Conference on Tobacco and Health; 1990 Apr 1‐5; Perth, Australia. 1990.
Engeln 1969 {published data only}
-
- Engeln RG. A comparison of desensitization and aversive conditioning as treatment methods to reduce cigarette smoking [thesis]. USA: Dissertation Abstracts International, 1969.
Gardner 1971 {published data only}
-
- Gardner RM. A Test of Coverant Control Therapy to Reduce Cigarette Smoking: A Comparative Study of the Effectiveness of Two Different Strategies with a Direct Test of the Effectiveness of Contingency Management [thesis]. USA: Dissertation Abstracts International, 1971.
Palmer 1983 {published data only}
-
- Palmer RD. A Multiple Stage Treatment for Smoking Reduction [thesis]. USA: Dissertation Abstracts International, 1983.
Rennard 1994 {published data only}
-
- Rennard SI, Daughton DM, Thompson AB, Floreani AA, Romberger DJ, Millatmal T. The effects of nicotine replacement therapy on cigarette smoking reduction. Annual Meeting of the American Thoracic Association; 1994; Boston, USA. 1994.
Weis 1974 {published data only}
-
- Weis JI. An Experimental Examination of Cautela's Covert Sensitization as a Smoking Reduction Technique [thesis]. USA: Dissertation Abstracts International, 1974.
References to ongoing studies
Hagens 2017 {published data only}
-
- Hagens P, Pieterse M, Valk P, Palen J. Effectiveness of intensive smoking reduction counselling plus combination nicotine replacement therapy in promoting long‐term abstinence in patients with chronic obstructive pulmonary disease not ready to quit smoking: protocol of the REDUQ trial. Contemporary Clinical Trials Communications 2017;8:248‐57. - PMC - PubMed
-
- NTR2227. Effects of a reduction‐to‐quit smoking programme in patients with COPD: the REDUQ study [A randomised controlled trial with 18 months follow‐up in COPD patients comparing a smoking reduction programme with a self‐help intervention, on reduction followed by sustained cessation]. www.trialregister.nl/trial/2110 (first received 2 February 2010).
NCT02124187 {published data only}
-
- NCT02124187. Smoking cessation and reduction in depression. clinicaltrials.gov/ct2/show/NCT02124187 (first received 28 April 2014).
NCT02354872 {published data only}
-
- NCT02354872. Motivation project: testing intervention components for the smoker who is unwilling to quit. clinicaltrials.gov/ct2/show/NCT02354872 (first received 3 February 2015).
NCT02370147 {published data only}
-
- NCT02370147. Very brief smoking reduction intervention. clinicaltrials.gov/ct2/show/NCT02370147 (first received 24 February 2015).
NCT02515500 {published data only}
-
- NCT02515500. Cigarette reduction using the quitbit digital lighter and mobile application for smoking cessation: a randomized controlled trial. clinicaltrials.gov/ct2/show/NCT02515500 (first received 4 August 2015).
NCT02564315 {published data only}
-
- NCT02564315. The long‐term quitting (smoking cessation) study. clinicaltrials.gov/ct2/show/NCT02564315 (first received 30 September 2015).
NCT02894957 {published data only}
-
- NCT02894957. Varenicline for "gradual" vs. "abrupt" smoking cessation in low‐motivated COPD smokers. clinicaltrials.gov/ct2/show/NCT02894957 (first received 9 September 2016).
NCT02905656 {published data only}
-
- NCT02905656. Strategies to promote cessation in smokers who are not ready to quit. clinicaltrials.gov/ct2/show/NCT02905656 (first received 19 September 2016).
Additional references
Bandura 1977
-
- Bandura A. Self efficacy: toward a unifying theory of behavioral change. Psychological Review 1977;84(2):191‐215. - PubMed
Bouton 1991
-
- Bouton ME, Swartzentruber D. Sources of relapse after extinction in Pavlovian and instrumental learning. Clinical Psychology Review 1991;11(2):123‐40. [DOI: ]
Cahill 2016
CDC 2017
-
- Centers for Disease Control and Prevention. Quitting smoking among adults—United States, 2000–2015. Morbidity and Mortality Weekly Report 2017; Vol. 65, issue 52:1457‐64. - PubMed
Chaiton 2016
Chamberlain 2017
Cheong 2007
-
- Cheong Y, Yong H, Borland R. Does how you quit affect success? A comparison between abrupt and gradual methods using data from the international tobacco control policy evaluation study. Nicotine & Tobacco Research 2007;9(8):801‐10. - PubMed
Cohen 1983
-
- Cohen S, Hobermann HM. Positive events and social supports as buffers of life change stress. Journal of Applied Social Psychology 1983;13:99‐125.
Denning 2002
-
- Denning P. Harm reduction psychotherapy: an innovative alternative to classical addictions theory. American Clinical Laboratory 2002;21(4):16‐8. - PubMed
Etter 2005
-
- Etter JF. A self‐administered questionnaire to measure cigarette withdrawal symptoms: the Cigarette Withdrawal Scale. Nicotine & Tobacco Research 2005;7(1):47‐57. - PubMed
Fagerström 2002b
-
- Fagerström KO, Hughes JR. Nicotine concentrations with concurrent use of cigarettes and nicotine replacement: A review. Nicotine & Tobacco Research 2002;4((Suppl 2)):S73‐9. - PubMed
Fagerström 2005
Fiore 2008
-
- Fiore MC, Jaen CR, Baker TB, Bailey WC, Benowitz NL, Curry SJ, et al. Treating tobacco use and dependence: 2008 update. Available at www.ncbi.nlm.nih.gov/books/NBK63952/ 2008.
Garnett 2019
-
- Garnett C, Shahab L, Raupach T, West R, Brown J. Understanding the Association Between Spontaneous Quit Attempts and Improved Smoking Cessation Success Rates: A Population Survey in England With 6‐Month Follow‐up. Nicotine & Tobacco Research 2019;epub before print. [DOI: 10.1093/ntr/ntz115] - DOI - PMC - PubMed
GBD 2015 Risk Factors Collaborators 2016
-
- GBD 2015 Risk Factors Collaborators. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016;388:1659–724. [DOI: ] - PMC - PubMed
Hajek 1989
-
- Hajek P. Withdrawal‐oriented therapy for smokers. British Journal of Addiction 1989;84:591‐8. - PubMed
Hartmann‐Boyce 2018
Higgins 2011
-
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. Cochrane Collaboration.
Higgins 2017
-
- Higgins JP, Altman DG, Sterne JA, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Churchill R, Chandler J, Cumpston MS, editor(s), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), The Cochrane Collaboration, 2017. Available from www.training.cochrane.org/handbook.
Hughes 1986
-
- Hughes JR, Hatsukami D. Signs and symptoms of tobacco withdrawal. Archives of General Psychiatry 1986;43:289‐94. - PubMed
Hughes 2004a
-
- Hughes JR, Keely J, Naud S. Shape of the relapse curve and long‐term abstinence among untreated smokers. Addiction 2004;99(1):29‐38. [DOI: ] - PubMed
Hughes 2006
Hughes 2014
Jha 2011
-
- Jha P. Avoidable deaths from smoking: a global perspective. Public Health Reviews 2011;33(2):569‐600.
Lancaster 2017
Lindson 2012
-
- Lindson N. Smoking reduction and nicotine preloading: new approaches to cessation? [PhD thesis]. Available at www.etheses.bham.ac.uk/3245/1/Lindson12PhD.pdf 2012.
Lindson 2019
Lindson‐Hawley 2016a
Livingstone‐Banks 2019
McRobbie 2006
-
- McRobbie H, Whittaker R, Bullen C. Using nicotine replacement therapy to assist in reducing cigarette consumption before quitting: another strategy for smoking cessation?. Disease Management and Health Outcomes 2006;4(6):335‐40.
Moore 2009
NICE 2018
-
- National Institute for Health and Care Excellence. Stop smoking interventions and services. NICE guideline [NG92]. www.nice.org.uk/guidance/ng92 2018 (Accessed 21 May 2018).
Peters 2007
-
- Peters EN, Hughes JR, Callas PW, Solomon LJ. Goals indicate motivation to quit smoking. Addiction 2007;102(7):1158‐63. [DOI: ] - PubMed
Schünemann 2017
-
- Schünemann HJ, Oxman AD, Higgins JP, Vist GE, Glasziou P, Akl E, et al on behalf of the Cochrane GRADEing Methods Group and the Cochrane Statistical Methods Group. Chapter 11: Completing ‘Summary of findings’ tables and grading the confidence in or quality of the evidence. In: Higgins JP, Churchill R, Chandler J, Cumpston MS, editor(s), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017). The Cochrane Collaboration, 2017. Available from www.training.cochrane.org/handbook.
Shiffman 2007
-
- Shiffman S, Hughes JR, Ferguson SG, Pillitteri JL, Gitchell JG, Burton SL. Smokers' interest in using nicotine replacement to aid smoking reduction. Nicotine & Tobacco Research 2007;9(11):1177‐82. - PubMed
Skinner 1953
-
- Skinner BF. Science and Human Behavior. Oxford: MacMillan, 1953.
Stead 2017
Wang 2008
-
- Wang D, Connock M, Barton P, Fry‐Smith A, Aveyard P, Moore D. 'Cut down to quit' with nicotine replacement therapies in smoking cessation: a systematic review of effectiveness and economic analysis. Health Technology Assessment 2008;12(2):1‐135. - PubMed
West 2005
West 2006
-
- West 2006. Smoking and smoking cessation in England: 2006. 66.102.1.104/scholar?hl=en&lr=&client=firefox‐a&q=cache:UDuwOCSGZssJ:www.aspsilverbackwebsites.co.uk/smokinginengland/Ref/paper4.pdf+%22smoki... 2006.
West 2012
-
- West R, Brown J. Smoking and smoking cessation in England 2011: Findings from the smoking toolkit study. http://www.minerva‐ebm.be/Resource/Get/10033 (accessed 12 September 2019).
Wu 2015
-
- Wu L, Sun S, He Y, Zeng J. Effect of smoking reduction therapy on smoking cessation for smokers without an intention to quit: an updated systematic review and meta‐analysis of randomized controlled trials. International Journal of Environmental Research and Public Health 2015;12(9):10235‐53. [DOI: 10.3390/ijerph120910235] - DOI - PMC - PubMed
References to other published versions of this review
Lindson 2009
Lindson 2010
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

