Inhibiting Analyte Theft in Surface-Enhanced Raman Spectroscopy Substrates: Subnanomolar Quantitative Drug Detection
- PMID: 31565921
- PMCID: PMC6878213
- DOI: 10.1021/acssensors.9b01484
Inhibiting Analyte Theft in Surface-Enhanced Raman Spectroscopy Substrates: Subnanomolar Quantitative Drug Detection
Abstract
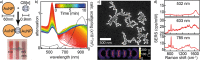
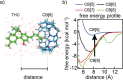
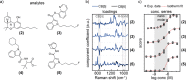
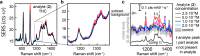
Quantitative applications of surface-enhanced Raman spectroscopy (SERS) often rely on surface partition layers grafted to SERS substrates to collect and trap-solvated analytes that would not otherwise adsorb onto metals. Such binding layers drastically broaden the scope of analytes that can be probed. However, excess binding sites introduced by this partition layer also trap analytes outside the plasmonic "hotspots". We show that by eliminating these binding sites, limits of detection (LODs) can effectively be lowered by more than an order of magnitude. We highlight the effectiveness of this approach by demonstrating quantitative detection of controlled drugs down to subnanomolar concentrations in aqueous media. Such LODs are low enough to screen, for example, urine at clinically relevant levels. These findings provide unique insights into the binding behavior of analytes, which are essential when designing high-performance SERS substrates.
Keywords: SERS; THC; drug detection; nanoparticles; self-assembly; spice; synthetic cannabinoids; tetrahydrocannabinol.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Sharma B.; Fernanda Cardinal M.; Kleinman S. L.; Greeneltch N. G.; Frontiera R. R.; Blaber M. G.; Schatz G. C.; Van Duyne R. P. High-Performance SERS Substrates: Advances and Challenges. MRS Bull. 2013, 38, 615–624. 10.1557/mrs.2013.161. - DOI
-
- Sharma B.; Frontiera R. R.; Henry A.-I.; Ringe E.; Van Duyne R. P. SERS: Materials, Applications, and the Future. Mater. Today 2012, 15, 16–25. 10.1016/s1369-7021(12)70017-2. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

