Impaired NF-κB signalling underlies cyclophilin D-mediated mitochondrial permeability transition pore opening in doxorubicin cardiomyopathy
- PMID: 31566215
- PMCID: PMC7177490
- DOI: 10.1093/cvr/cvz240
Impaired NF-κB signalling underlies cyclophilin D-mediated mitochondrial permeability transition pore opening in doxorubicin cardiomyopathy
Abstract
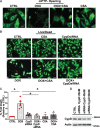
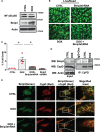
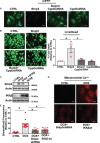
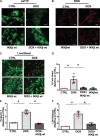
Aims: The chemotherapy drug doxorubicin (Dox) is commonly used for treating a variety of human cancers; however, it is highly cardiotoxic and induces heart failure. We previously reported that the Bcl-2 mitochondrial death protein Bcl-2/19kDa interaction protein 3 (Bnip3), is critical for provoking mitochondrial perturbations and necrotic cell death in response to Dox; however, the underlying mechanisms had not been elucidated. Herein, we investigated mechanism that drives Bnip3 gene activation and downstream effectors of Bnip3-mediated mitochondrial perturbations and cell death in cardiac myocytes treated with Dox.
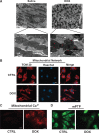
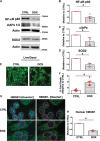
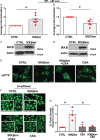
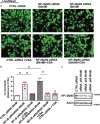
Methods and results: Nuclear factor-κB (NF-κB) signalling, which transcriptionally silences Bnip3 activation under basal states in cardiac myocytes was dramatically reduced following Dox treatment. This was accompanied by Bnip3 gene activation, mitochondrial injury including calcium influx, permeability transition pore (mPTP) opening, loss of nuclear high mobility group protein 1, reactive oxygen species production, and cell death. Interestingly, impaired NF-κB signalling in cells treated with Dox was accompanied by protein complexes between Bnip3 and cyclophilin D (CypD). Notably, Bnip3-mediated mPTP opening was suppressed by inhibition of CypD-demonstrating that CypD functionally operates downstream of Bnip3. Moreover, restoring IKKβ-NF-κB activity in cardiac myocytes treated with Dox suppressed Bnip3 expression, mitochondrial perturbations, and necrotic cell death.
Conclusions: The findings of the present study reveal a novel signalling pathway that functionally couples NF-κB and Dox cardiomyopathy to a mechanism that is mutually dependent upon and obligatorily linked to the transcriptional control of Bnip3. Our findings further demonstrate that mitochondrial injury and necrotic cell death induced by Bnip3 is contingent upon CypD. Hence, maintaining NF-κB signalling may prove beneficial in reducing mitochondrial dysfunction and heart failure in cancer patients undergoing Dox chemotherapy.
Keywords: Bnip3; Cardiac myocytes; Cell death; Doxorubicin; Mitochondria; NF-κB.
Published on behalf of the European Society of Cardiology. All rights reserved. © The Author(s) 2019. For permissions, please email: journals.permissions@oup.com.
Figures

Comment in
-
Mitochondrial therapy for doxorubicin cardiomyopathy: nuclear factor-κB to the rescue?Cardiovasc Res. 2020 May 1;116(6):1092-1094. doi: 10.1093/cvr/cvz344. Cardiovasc Res. 2020. PMID: 31868877 Free PMC article. No abstract available.
Similar articles
-
Bnip3 mediates doxorubicin-induced cardiac myocyte necrosis and mortality through changes in mitochondrial signaling.Proc Natl Acad Sci U S A. 2014 Dec 23;111(51):E5537-44. doi: 10.1073/pnas.1414665111. Epub 2014 Dec 8. Proc Natl Acad Sci U S A. 2014. PMID: 25489073 Free PMC article.
-
Ellagic acid antagonizes Bnip3-mediated mitochondrial injury and necrotic cell death of cardiac myocytes.Free Radic Biol Med. 2017 Nov;112:411-422. doi: 10.1016/j.freeradbiomed.2017.08.010. Epub 2017 Aug 30. Free Radic Biol Med. 2017. PMID: 28838842
-
Nuclear factor-kappaB-mediated cell survival involves transcriptional silencing of the mitochondrial death gene BNIP3 in ventricular myocytes.Circulation. 2005 Dec 13;112(24):3777-85. doi: 10.1161/CIRCULATIONAHA.105.573899. Circulation. 2005. PMID: 16344406
-
Cyclophilin D-mediated Mitochondrial Permeability Transition Regulates Mitochondrial Function.Curr Pharm Des. 2023;29(8):620-629. doi: 10.2174/1381612829666230313111314. Curr Pharm Des. 2023. PMID: 36915987 Review.
-
The beneficial role of exercise in mitigating doxorubicin-induced Mitochondrionopathy.Biochim Biophys Acta Rev Cancer. 2018 Apr;1869(2):189-199. doi: 10.1016/j.bbcan.2018.01.002. Epub 2018 Feb 14. Biochim Biophys Acta Rev Cancer. 2018. PMID: 29408395 Review.
Cited by
-
Isoquercitrin alleviates pirarubicin-induced cardiotoxicity in vivo and in vitro by inhibiting apoptosis through Phlpp1/AKT/Bcl-2 signaling pathway.Front Pharmacol. 2024 Mar 18;15:1315001. doi: 10.3389/fphar.2024.1315001. eCollection 2024. Front Pharmacol. 2024. PMID: 38562460 Free PMC article.
-
Molecular mechanisms of doxorubicin-induced cardiotoxicity: novel roles of sirtuin 1-mediated signaling pathways.Cell Mol Life Sci. 2021 Apr;78(7):3105-3125. doi: 10.1007/s00018-020-03729-y. Epub 2021 Jan 13. Cell Mol Life Sci. 2021. PMID: 33438055 Free PMC article. Review.
-
Therapeutic Targets for Regulating Oxidative Damage Induced by Ischemia-Reperfusion Injury: A Study from a Pharmacological Perspective.Oxid Med Cell Longev. 2022 Apr 11;2022:8624318. doi: 10.1155/2022/8624318. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 35450409 Free PMC article. Review.
-
GAS-STING signaling plays an essential pathogenetic role in Doxorubicin-Induced Cardiotoxicity.BMC Pharmacol Toxicol. 2023 Mar 24;24(1):19. doi: 10.1186/s40360-022-00631-0. BMC Pharmacol Toxicol. 2023. PMID: 36964634 Free PMC article.
-
Mitochondrial therapy for doxorubicin cardiomyopathy: nuclear factor-κB to the rescue?Cardiovasc Res. 2020 May 1;116(6):1092-1094. doi: 10.1093/cvr/cvz344. Cardiovasc Res. 2020. PMID: 31868877 Free PMC article. No abstract available.
References
-
- Ducut Sigala JL, Bottero V, Young DB, Shevchenko A, Mercurio F, Verma IM.. Activation of transcription factor NF-kappaB requires ELKS, an IkappaB kinase regulatory subunit. Science 2004;304:1963–1967. - PubMed
-
- Baeuerle P, Baltimore D.. I kappa B: a specific inhibitor of the NF-kappa B transcription factor. Science 1988;242:540–546. - PubMed
-
- Chen Z, Hagler J, Palombella VJ, Melandri F, Scherer D, Ballard D, Maniatis T.. Signal-induced site-specific phosphorylation targets I kappa B alpha to the ubiquitin-proteasome pathway. Genes Dev 1995;9:1586–1597. - PubMed
-
- Ghosh S, Karin M.. Missing pieces in the NF-kappaB puzzle. Cell 2002;109 Suppl:S81–S96. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

