Development of Chemical Entities Endowed with Potent Fast-Killing Properties against Plasmodium falciparum Malaria Parasites
- PMID: 31566384
- PMCID: PMC6816013
- DOI: 10.1021/acs.jmedchem.9b01099
Development of Chemical Entities Endowed with Potent Fast-Killing Properties against Plasmodium falciparum Malaria Parasites
Abstract
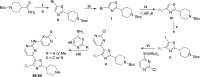
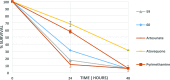
One of the attractive properties of artemisinins is their extremely fast-killing capability, quickly relieving malaria symptoms. Nevertheless, the unique benefits of these medicines are now compromised by the prolonged parasite clearance times and the increasing frequency of treatment failures, attributed to the increased tolerance of Plasmodium falciparum to artemisinin. This emerging artemisinin resistance threatens to undermine the effectiveness of antimalarial combination therapies. Herein, we describe the medicinal chemistry efforts focused on a cGMP-dependent protein kinase (PKG) inhibitor scaffold, leading to the identification of novel chemical entities with very potent, similar to artemisinins, fast-killing potency against asexual blood stages that cause disease, and activity against gametocyte activation that is required for transmission. Furthermore, we confirm that selective PKG inhibitors have a slow speed of kill, while chemoproteomic analysis suggests for the first time serine/arginine protein kinase 2 (SRPK2) targeting as a novel strategy for developing antimalarial compounds with extremely fast-killing properties.
Conflict of interest statement
The authors declare no competing financial interest.
Figures






Similar articles
-
Inhibition of Resistance-Refractory P. falciparum Kinase PKG Delivers Prophylactic, Blood Stage, and Transmission-Blocking Antiplasmodial Activity.Cell Chem Biol. 2020 Jul 16;27(7):806-816.e8. doi: 10.1016/j.chembiol.2020.04.001. Epub 2020 Apr 30. Cell Chem Biol. 2020. PMID: 32359426 Free PMC article.
-
Imidazopyridazine Inhibitors of Plasmodium falciparum Calcium-Dependent Protein Kinase 1 Also Target Cyclic GMP-Dependent Protein Kinase and Heat Shock Protein 90 To Kill the Parasite at Different Stages of Intracellular Development.Antimicrob Agents Chemother. 2015 Dec 28;60(3):1464-75. doi: 10.1128/AAC.01748-15. Antimicrob Agents Chemother. 2015. PMID: 26711771 Free PMC article.
-
Trisubstituted thiazoles as potent and selective inhibitors of Plasmodium falciparum protein kinase G (PfPKG).Bioorg Med Chem Lett. 2018 Oct 15;28(19):3168-3173. doi: 10.1016/j.bmcl.2018.08.028. Epub 2018 Aug 27. Bioorg Med Chem Lett. 2018. PMID: 30174152 Free PMC article.
-
A brief history of artemisinin: Modes of action and mechanisms of resistance.Chin J Nat Med. 2019 May 20;17(5):331-336. doi: 10.1016/S1875-5364(19)30038-X. Chin J Nat Med. 2019. PMID: 31171267 Review.
-
Biological actions of artemisinin: insights from medicinal chemistry studies.Molecules. 2010 Mar 8;15(3):1378-97. doi: 10.3390/molecules15031378. Molecules. 2010. PMID: 20335987 Free PMC article. Review.
Cited by
-
Discovery of potent Plasmodium falciparum protein kinase 6 (PfPK6) inhibitors with a type II inhibitor pharmacophore.Eur J Med Chem. 2023 Mar 5;249:115043. doi: 10.1016/j.ejmech.2022.115043. Epub 2022 Dec 30. Eur J Med Chem. 2023. PMID: 36736152 Free PMC article.
-
Plasmodium falciparum cGMP-Dependent Protein Kinase - A Novel Chemotherapeutic Target.Front Microbiol. 2021 Feb 3;11:610408. doi: 10.3389/fmicb.2020.610408. eCollection 2020. Front Microbiol. 2021. PMID: 33613463 Free PMC article. Review.
-
Discovery of isoxazolyl-based inhibitors of Plasmodium falciparum cGMP-dependent protein kinase.RSC Med Chem. 2019 Dec 16;11(1):98-101. doi: 10.1039/c9md00511k. eCollection 2020 Jan 1. RSC Med Chem. 2019. PMID: 33479608 Free PMC article.
-
Chemoproteomics for Plasmodium Parasite Drug Target Discovery.Chembiochem. 2021 Aug 17;22(16):2591-2599. doi: 10.1002/cbic.202100155. Epub 2021 Jun 10. Chembiochem. 2021. PMID: 33999499 Free PMC article. Review.
-
Ca2+ signals critical for egress and gametogenesis in malaria parasites depend on a multipass membrane protein that interacts with PKG.Sci Adv. 2021 Mar 24;7(13):eabe5396. doi: 10.1126/sciadv.abe5396. Print 2021 Mar. Sci Adv. 2021. PMID: 33762339 Free PMC article.
References
-
- www.who.int/news-room/fact-sheets/detail/malaria (accessed February 25, 2019).
-
- Straimer J.; Gnädig N. F.; Witkowski B.; Amaratunga C.; Duru V.; Ramadani A. P.; Dacheux M.; Khim N.; Zhang L.; Lam S.; Gregory P. D.; Urnov F. D.; Mercereau-Puijalon O.; Benoit-Vical F.; Fairhurst R. M.; Mènard D.; Fidock D. A. Drug resistance. K13-propeller mutations confer artemisin resistance in Plasmodium falciparum clinical isolates. Science 2015, 347, 428–431. 10.1126/science.1260867. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Chemical Information

