Progression of diabetic kidney disease in T2DN rats
- PMID: 31566426
- PMCID: PMC6960784
- DOI: 10.1152/ajprenal.00246.2019
Progression of diabetic kidney disease in T2DN rats
Abstract
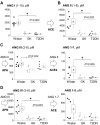
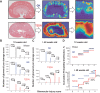
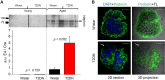
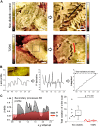
Diabetic kidney disease (DKD) is one of the leading pathological causes of decreased renal function and progression to end-stage kidney failure. To explore and characterize age-related changes in DKD and associated glomerular damage, we used a rat model of type 2 diabetic nephropathy (T2DN) at 12 wk and older than 48 wk. We compared their disease progression with control nondiabetic Wistar and diabetic Goto-Kakizaki (GK) rats. During the early stages of DKD, T2DN and GK animals revealed significant increases in blood glucose and kidney-to-body weight ratio. Both diabetic groups had significantly altered renin-angiotensin-aldosterone system function. Thereafter, during the later stages of disease progression, T2DN rats demonstrated a remarkable increase in renal damage compared with GK and Wistar rats, as indicated by renal hypertrophy, polyuria accompanied by a decrease in urine osmolarity, high cholesterol, a significant prevalence of medullary protein casts, and severe forms of glomerular injury. Urinary nephrin shedding indicated loss of the glomerular slit diaphragm, which also correlates with the dramatic elevation in albuminuria and loss of podocin staining in aged T2DN rats. Furthermore, we used scanning ion microscopy topographical analyses to detect and quantify the pathological remodeling in podocyte foot projections of isolated glomeruli from T2DN animals. In summary, T2DN rats developed renal and physiological abnormalities similar to clinical observations in human patients with DKD, including progressive glomerular damage and a significant decrease in renin-angiotensin-aldosterone system plasma levels, indicating these rats are an excellent model for studying the progression of renal damage in type 2 DKD.
Keywords: diabetic glomerular disease; diabetic nephropathy; podocyte pathology; renin-angiotensin-aldosterone system; scanning ion microscopy; type 2 diabetic nephropathy.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures



References
-
- Anguiano L, Andres H. Diabetic kidney disease. What we knew, what we know, and what we still do not know. ASN Kidney News 11: 8–9, 2019.
-
- Barnett AH, Mithal A, Manassie J, Jones R, Rattunde H, Woerle HJ, Broedl UC; EMPA-REG RENAL trial investigators . Efficacy and safety of empagliflozin added to existing antidiabetes treatment in patients with type 2 diabetes and chronic kidney disease: a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol 2: 369–384, 2014. doi: 10.1016/S2213-8587(13)70208-0. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

