Allosteric activation of the nitric oxide receptor soluble guanylate cyclase mapped by cryo-electron microscopy
- PMID: 31566566
- PMCID: PMC6839917
- DOI: 10.7554/eLife.50634
Allosteric activation of the nitric oxide receptor soluble guanylate cyclase mapped by cryo-electron microscopy
Abstract
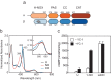
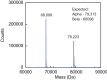
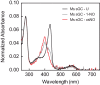
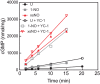
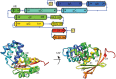
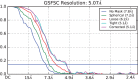
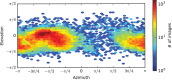
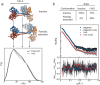
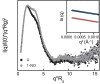
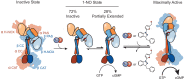
Soluble guanylate cyclase (sGC) is the primary receptor for nitric oxide (NO) in mammalian nitric oxide signaling. We determined structures of full-length Manduca sexta sGC in both inactive and active states using cryo-electron microscopy. NO and the sGC-specific stimulator YC-1 induce a 71° rotation of the heme-binding β H-NOX and PAS domains. Repositioning of the β H-NOX domain leads to a straightening of the coiled-coil domains, which, in turn, use the motion to move the catalytic domains into an active conformation. YC-1 binds directly between the β H-NOX domain and the two CC domains. The structural elongation of the particle observed in cryo-EM was corroborated in solution using small angle X-ray scattering (SAXS). These structures delineate the endpoints of the allosteric transition responsible for the major cyclic GMP-dependent physiological effects of NO.
Keywords: CryoEM; SAXS; allostery; biochemistry; chemical biology; cyclic GMP; manduca sexta; nitric oxide; none.
Conflict of interest statement
BH, AY, DR, KM, MH, JH No competing interests declared, MM Reviewing editor, eLife
Figures




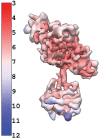








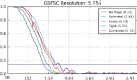

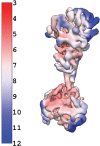
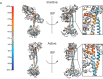



Comment in
-
Wedging open a catalytic site.Elife. 2019 Nov 8;8:e52418. doi: 10.7554/eLife.52418. Elife. 2019. PMID: 31701871 Free PMC article.
References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

