Diverse roles for CDK-associated activity during spermatogenesis
- PMID: 31566717
- PMCID: PMC6900092
- DOI: 10.1002/1873-3468.13627
Diverse roles for CDK-associated activity during spermatogenesis
Abstract
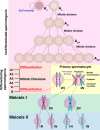
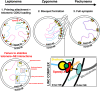
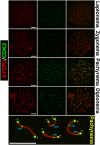
The primary function of cyclin-dependent kinases (CDKs) in complex with their activating cyclin partners is to promote mitotic division in somatic cells. This canonical cell cycle-associated activity is also crucial for fertility as it allows the proliferation and differentiation of stem cells within the reproductive organs to generate meiotically competent cells. Intriguingly, several CDKs exhibit meiosis-specific functions and are essential for the completion of the two reductional meiotic divisions required to generate haploid gametes. These meiosis-specific functions are mediated by both known CDK/cyclin complexes and meiosis-specific CDK-regulators and are important for a variety of processes during meiotic prophase. The majority of meiotic defects observed upon deletion of these proteins occur during the extended prophase I of the first meiotic division. Importantly a lack of redundancy is seen within the meiotic arrest phenotypes described for many of these proteins, suggesting intricate layers of cell cycle control are required for normal meiotic progression. Using the process of male germ cell development (spermatogenesis) as a reference, this review seeks to highlight the diverse roles of selected CDKs their activators, and their regulators during gametogenesis.
Keywords: cyclin; cyclin-dependent kinase; meiosis; meiotic crossover; recombination; synapsis.
© 2019 The Authors. FEBS Letters published by John Wiley & Sons Ltd on behalf of Federation of European Biochemical Societies.
Figures

References
-
- Morgan DO (1997) Cyclin‐dependent kinases: engines, clocks, and microprocessors. Annu Rev Cell Dev Biol 13, 261–291. - PubMed
-
- Doonan JH and Kitsios G (2009) Functional evolution of cyclin‐dependent kinases. Mol Biotechnol 42, 14–29. - PubMed
-
- Lim S and Kaldis P (2013) Cdks, cyclins and CKIs: roles beyond cell cycle regulation. Development 140, 3079–3093. - PubMed
-
- Loyer P, Trembley JH, Katona R, Kidd VJ and Lahti JM (2005) Role of CDK/cyclin complexes in transcription and RNA splicing. Cell Signal 17, 1033–1051. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

