Cryosurgical ablation for treatment of rhinitis: A prospective multicenter study
- PMID: 31566744
- PMCID: PMC7384004
- DOI: 10.1002/lary.28301
Cryosurgical ablation for treatment of rhinitis: A prospective multicenter study
Abstract
Objective: To assess the efficacy and safety of cryoablation of the posterior nasal nerve (PNN) for treatment of chronic rhinitis.
Methods: This was a prospective single-arm trial of 98 adult patients at six U.S. centers with chronic allergic and nonallergic rhinitis. PNN cryoablation was performed in-office under local anesthesia using a handheld device. Patients discontinued use of intranasal ipratropium 3 days prior to treatment and throughout the study period. Reflective Total Nasal Symptom Score (rTNSS) was measured at pretreatment baseline and posttreatment at 1 month, 3 months, 6 months, and 9 months. The Rhinoconjunctivitis Quality of Life Questionnaire (RQLQ) was completed at pretreatment and 3 months posttreatment. Adverse effects and postprocedure medication usage were recorded.
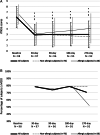
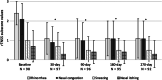
Results: Ninety-eight procedures (100%) were successfully completed. rTNSS significantly improved over pretreatment baseline (6.1 ± 1.9) at 1 month (2.9 ± 1.9, P < 0.001), 3 months (3.0 ± 2.3, P < 0.001), 6 months (3.0 ± 2.1, P < 0.001), and 9 months (3.0 ± 2.4, P < 0.001) postprocedure. Nasal congestion and rhinorrhea subscores improved significantly at all time points (P < 0.001). Both allergic and nonallergic rhinitis subcohorts showed improvement (P < 0.001), with a comparable degree of improvement between groups. RQLQ significantly improved over pretreatment baseline (3.0 ± 1.0) at 3 months (1.5 ± 1.0, P < 0.001), and all RQLQ subdomains demonstrated improvement. Of 54 patients using intranasal medication at baseline, 19 (35.2%) were able to discontinue use. Twenty-nine adverse effects were reported, including headache, epistaxis, and sinusitis.
Conclusion: Cryoablation of the PNN for chronic rhinitis is safe and can result in relief of nasal symptoms and improvements in quality of life.
Level of evidence: 4 Laryngoscope, 130: 1877-1884, 2020.
Trial registration: ClinicalTrials.gov NCT03181594.
Keywords: Rhinitis; congestion; cryoablation; cryosurgery; posterior nasal nerve; rhinorrhea.
© 2019 The Authors. The Laryngoscope published by Wiley Periodicals, Inc. on behalf of The American Laryngological, Rhinological and Otological Society, Inc.
Figures


Similar articles
-
Effectiveness of ClariFix (Cryoablation) of the Posterior Nasal Nerve on Nasal Symptoms in Patients With Chronic Rhinitis: A Systematic Review and Meta-Analysis.J Rhinol. 2024 Jul;31(2):57-66. doi: 10.18787/jr.2024.00015. Epub 2024 Jul 31. J Rhinol. 2024. PMID: 39664403 Free PMC article. Review.
-
Cryosurgical Ablation for Treatment of Rhinitis: Two-Year Results of a Prospective Multicenter Study.Laryngoscope. 2021 Sep;131(9):1952-1957. doi: 10.1002/lary.29453. Epub 2021 Feb 22. Laryngoscope. 2021. PMID: 33616224 Free PMC article. Clinical Trial.
-
Cryosurgical posterior nasal tissue ablation for the treatment of rhinitis.Int Forum Allergy Rhinol. 2017 Oct;7(10):952-956. doi: 10.1002/alr.21991. Epub 2017 Aug 11. Int Forum Allergy Rhinol. 2017. PMID: 28799727 Free PMC article. Clinical Trial.
-
Temperature-controlled radiofrequency ablation for the treatment of chronic rhinitis: Two-year outcomes from a prospective multicenter trial.Int Forum Allergy Rhinol. 2024 Jul;14(7):1182-1194. doi: 10.1002/alr.23315. Epub 2024 Jan 24. Int Forum Allergy Rhinol. 2024. PMID: 38266636 Clinical Trial.
-
Effectiveness of the Posterior Nasal Nerve Cryoablation in Allergic and Non-Allergic Rhinitis.Laryngoscope. 2024 Jun;134(6):2502-2512. doi: 10.1002/lary.31163. Epub 2023 Nov 22. Laryngoscope. 2024. PMID: 37991147 Review.
Cited by
-
Patient Questions Surrounding Posterior Nasal Nerve Ablation for Chronic Rhinitis.OTO Open. 2024 Jun 6;8(2):e156. doi: 10.1002/oto2.156. eCollection 2024 Apr-Jun. OTO Open. 2024. PMID: 38846014 Free PMC article.
-
Clinical and Quality of Life Outcomes Following Temperature-Controlled Radiofrequency Neurolysis of the Posterior Nasal Nerve (RhinAer) for Treatment of Chronic Rhinitis.Am J Rhinol Allergy. 2022 Nov;36(6):747-754. doi: 10.1177/19458924221109987. Epub 2022 Jul 11. Am J Rhinol Allergy. 2022. PMID: 35818709 Free PMC article.
-
Effect of Endoscopic Posterior Nasal Neurectomy on Laryngectomy-associated Rhinorrhea: A Feasibility Study.In Vivo. 2023 Nov-Dec;37(6):2648-2653. doi: 10.21873/invivo.13373. In Vivo. 2023. PMID: 37905635 Free PMC article.
-
Clinical evaluation of a novel multipoint radiofrequency ablation device to treat chronic rhinitis.Laryngoscope Investig Otolaryngol. 2023 Mar 16;8(2):367-372. doi: 10.1002/lio2.1040. eCollection 2023 Apr. Laryngoscope Investig Otolaryngol. 2023. PMID: 37090860 Free PMC article.
-
Effectiveness of ClariFix (Cryoablation) of the Posterior Nasal Nerve on Nasal Symptoms in Patients With Chronic Rhinitis: A Systematic Review and Meta-Analysis.J Rhinol. 2024 Jul;31(2):57-66. doi: 10.18787/jr.2024.00015. Epub 2024 Jul 31. J Rhinol. 2024. PMID: 39664403 Free PMC article. Review.
References
-
- Settipane RA, Charnock DR. Epidemiology of rhinitis: allergic and nonallergic. Clin Allergy Immunol 2007;19:23–34. - PubMed
-
- Lieberman P, Kaliner MA, Wheeler WJ. Open‐label evaluation of azelastine nasal spray in patients with seasonal allergic rhinitis and nonallergic vasomotor rhinitis. Curr Med Res Opin 2005;21:611–618. - PubMed
-
- Golding‐Wood PH. Observations on petrosal and vidian neurectomy in chronic vasomotor rhinitis. J Laryngol Otol 1961;75:232–247. - PubMed
-
- Kanaya T, Kikawada T. Endoscopic posterior nasal neurectomy: an alternative to Vidian neurectomy. Clin Exp Allergy Rev 2009;9:24–27.
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

