A Method for High-Throughput Measurements of Viscosity in Sub-micrometer-Sized Membrane Systems
- PMID: 31566864
- PMCID: PMC7154536
- DOI: 10.1002/cbic.201900510
A Method for High-Throughput Measurements of Viscosity in Sub-micrometer-Sized Membrane Systems
Abstract
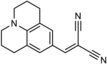
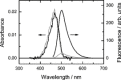
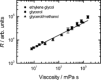
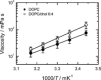
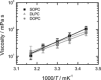
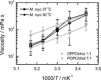
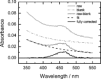
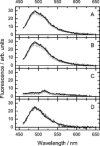
To unravel the underlying principles of membrane adaptation in small systems like bacterial cells, robust approaches to characterize membrane fluidity are needed. Currently available relevant methods require advanced instrumentation and are not suitable for high-throughput settings needed to elucidate the biochemical pathways involved in adaptation. We developed a fast, robust, and financially accessible quantitative method to measure the microviscosity of lipid membranes in bulk suspension using a commercially available plate reader. Our approach, which is suitable for high-throughput screening, is based on the simultaneous measurements of absorbance and fluorescence emission of a viscosity-sensitive fluorescent dye, 9-(2,2-dicyanovinyl)julolidine (DCVJ), incorporated into a lipid membrane. We validated our method using artificial membranes with various lipid compositions over a range of temperatures and observed values that were in good agreement with previously published results. Using our approach, we were able to detect a lipid phase transition in the ruminant pathogen Mycoplasma mycoides.
Keywords: bacteria; high-throughput screening; lipid membranes; spectroscopy; viscosity.
© 2019 The Authors. Published by Wiley-VCH Verlag GmbH & Co. KGaA.
Conflict of interest statement
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

