Critical considerations for targeting colorectal liver metastases with nanotechnology
- PMID: 31566913
- PMCID: PMC7027529
- DOI: 10.1002/wnan.1588
Critical considerations for targeting colorectal liver metastases with nanotechnology
Abstract
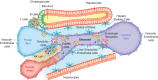
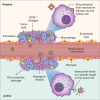
Colorectal cancer remains a significant cause of morbidity and mortality worldwide. Half of all patients develop liver metastases, presenting unique challenges for their treatment. The shortcomings of conventional chemotherapy has encouraged the use of nanomedicines; the application of nanotechnology in the diagnosis and treatment of disease. In spite of technological improvements in nanotechnology, the complexity of biological systems hinders the prospect of nanomedicines being applied in cancer therapy at the present time. This review highlights current biological barriers and discusses aspects of tumor biology together with the physicochemical features of the nanocarrier, that need to be considered in order to develop effective nanotherapeutics for colorectal cancer patients with liver metastases. It becomes clear that incorporating an interdisciplinary approach when developing nanomedicines should assure appropriate disease-driven design and that this will form a critical step in improving their clinical translation. This article is characterized under: Therapeutic Approaches and Drug Discovery > Nanomedicine for Oncologic Disease.
Keywords: colorectal cancer; drug delivery; liver metastases; nanomedicines; tumor biology.
© 2019 The Authors. WIREs Nanomedicine and Nanobiotechnology published by Wiley Periodicals, Inc.
Conflict of interest statement
A.O. and S.P.R. are co‐inventors of patents relating to drug delivery and are directors for Tandem Nano Ltd. The authors have declared no other conflicts of interest for this article.
Figures

References
-
- Adam, R. , de Gramont, A. , Figueras, J. , Kokudo, N. , Kunstlinger, F. , Loyer, E. , … of the EGOSLIM . (2015). Managing synchronous liver metastases from colorectal cancer: A multidisciplinary international consensus. Cancer Treatment Reviews, 41(9), 729–741. 10.1016/j.ctrv.2015.06.006 - DOI - PubMed
-
- Adua, D. , Di Fabio, F. , Ercolani, G. , Fiorentino, M. , Gruppioni, E. , Altimari, A. , … Pinto, C. (2017). Heterogeneity in the colorectal primary tumor and the synchronous resected liver metastases prior to and after treatment with an anti‐EGFR monoclonal antibody. Molecular and Clinical Oncology, 7(1), 113–120. 10.3892/mco.2017.1270 - DOI - PMC - PubMed
-
- Aird, W. C. (2007). Phenotypic heterogeneity of the endothelium: II. Representative vascular beds. Circulation Research, 100(2), 174–190. 10.1161/01.RES.0000255690.03436.ae - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

