Mortality Assessment of Paclitaxel-Coated Balloons: Patient-Level Meta-Analysis of the ILLUMENATE Clinical Program at 3 Years
- PMID: 31567024
- PMCID: PMC6784772
- DOI: 10.1161/CIRCULATIONAHA.119.040518
Mortality Assessment of Paclitaxel-Coated Balloons: Patient-Level Meta-Analysis of the ILLUMENATE Clinical Program at 3 Years
Abstract
Background: A recent summary-level meta-analysis comprising randomized, controlled trials (RCTs) of femoropopliteal paclitaxel-coated balloon and stent intervention identified excess late mortality in the paclitaxel-treated patients.
Methods: We evaluated the safety of the Stellarex drug-coated balloon (DCB) for femoropopliteal artery disease with an independently performed meta-analysis of patient-level data from all patients in the Stellarex femoropopliteal clinical program. To compare mortality after DCB or uncoated percutaneous transluminal angioplasty (PTA), we aggregated data from 2 RCTs comprising 419 patients treated with DCB and 170 patients treated with PTA. In an additional analysis, data were aggregated from 6 poolable Stellarex DCB studies (2 RCTs, 3 single-arm studies, and 1 registry). All serious adverse events including deaths were adjudicated by a blinded, third-party, independent Clinical Events Committee. Kaplan-Meier estimates in the RCTs were compared with restricted mean survival time. Predictors of death were assessed with hazard ratios (HRs) and Cox proportional hazards modeling.
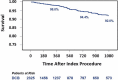
Results: Baseline characteristics were similar in the patients treated with DCB and PTA in the pooled RCT analysis, with the exception that the DCB cohort was younger (67.4±9.7 versus 69.4±9.4 years, P=0.02), smoked more frequently (86.6% versus 78.8%, P=0.02), and were less often treated for recurrent lesions (8.8% versus 14.7%, P=0.04). In the RCTs, patients treated with DCB had all-cause mortality rates that were not different from those of patients treated with PTA (Kaplan-Meier estimates 1.8±0.7% versus 1.3±0.9%, 6.5±1.2% versus 5.9±1.9%, and 9.3±1.5% versus 9.9±2.4% at 1, 2, and 3 years, respectively, P=0.86). All-cause mortality rates were similar in a 1906-patient pooled nonrandomized DCB data set (Kaplan-Meier estimates of 2.1%, 4.9%, and 7.0% at 1, 2, and 3 years, respectively). Clinical Events Committee-adjudicated causes of death were balanced between the DCB and PTA cohorts. Multivariable Cox modeling identified age (HR, 1.06; 95% CI, 1.04-1.08; P<0.001), diabetes mellitus (HR, 1.42; 95% CI, 1.01-2.00; P=0.04), congestive heart failure (HR, 1.88; 95% CI, 1.12-3.16; P=0.02), and renal insufficiency (HR, 2.00; 95% CI, 1.33-3.01; P<0.001) as predictors of mortality. Paclitaxel exposure was unrelated to mortality (HR, 1.04; 95% CI, 0.98-1.10; P=0.23).
Conclusions: The mortality rates for patients treated with the DCB and uncoated PTA were indistinguishable over 3-year follow-up. Additional patient-level, adequately powered meta-analyses with larger RCT data sets will be needed to confirm the generalizability of these findings.
Clinical trial registration: URL: http://www.clinicaltrials.gov. Unique identifiers: NCT02110524, NCT01858363, NCT01858428, NCT03421561, NCT01912937, NCT01927068, and NCT02769273.
Keywords: mortality; paclitaxel; peripheral vascular diseases.
Figures




Similar articles
-
Four-year patient-level pooled mortality analysis of the ILLUMENATE US Pivotal and EU randomized controlled trials.J Vasc Surg. 2022 Feb;75(2):600-607. doi: 10.1016/j.jvs.2021.07.244. Epub 2021 Sep 8. J Vasc Surg. 2022. PMID: 34506898 Review.
-
Stellarex Drug-Coated Balloon for Treatment of Femoropopliteal Disease: Twelve-Month Outcomes From the Randomized ILLUMENATE Pivotal and Pharmacokinetic Studies.Circulation. 2017 Sep 19;136(12):1102-1113. doi: 10.1161/CIRCULATIONAHA.117.028893. Epub 2017 Jul 20. Circulation. 2017. PMID: 28729250 Free PMC article. Clinical Trial.
-
Safety of Paclitaxel-Coated Balloon Angioplasty for Femoropopliteal Peripheral Artery Disease.JACC Cardiovasc Interv. 2019 Dec 23;12(24):2515-2524. doi: 10.1016/j.jcin.2019.08.025. Epub 2019 Sep 28. JACC Cardiovasc Interv. 2019. PMID: 31575518
-
Low-Dose Paclitaxel-Coated Versus Uncoated Percutaneous Transluminal Balloon Angioplasty for Femoropopliteal Peripheral Artery Disease: One-Year Results of the ILLUMENATE European Randomized Clinical Trial (Randomized Trial of a Novel Paclitaxel-Coated Percutaneous Angioplasty Balloon).Circulation. 2017 Jun 6;135(23):2227-2236. doi: 10.1161/CIRCULATIONAHA.116.026493. Epub 2017 Apr 19. Circulation. 2017. PMID: 28424223 Free PMC article. Clinical Trial.
-
Mortality Not Correlated With Paclitaxel Exposure: An Independent Patient-Level Meta-Analysis of a Drug-Coated Balloon.J Am Coll Cardiol. 2019 May 28;73(20):2550-2563. doi: 10.1016/j.jacc.2019.01.013. Epub 2019 Jan 25. J Am Coll Cardiol. 2019. PMID: 30690141 Review.
Cited by
-
Paclitaxel exposure: Long-term safety and effectiveness of a drug-coated balloon for claudication in pooled randomized trials.Catheter Cardiovasc Interv. 2020 Nov;96(5):1087-1099. doi: 10.1002/ccd.29152. Epub 2020 Aug 24. Catheter Cardiovasc Interv. 2020. PMID: 32830913 Free PMC article.
-
Drug-Coated Balloon versus Drug-Eluting Stent: The Debate of Leave Nothing Behind.Semin Intervent Radiol. 2023 Jun 16;40(2):161-166. doi: 10.1055/s-0043-57261. eCollection 2023 Apr. Semin Intervent Radiol. 2023. PMID: 37333737 Free PMC article. Review.
-
Single-Center Study Evaluating Long-Term Major Adverse Outcomes with the Use of Paclitaxel-Coated Balloons in Treating Infrainguinal Arterial Disease.Int J Angiol. 2023 Jan 13;32(1):48-55. doi: 10.1055/s-0042-1759818. eCollection 2023 Mar. Int J Angiol. 2023. PMID: 36727154 Free PMC article.
-
Data sources and applied methods for paclitaxel safety signal discernment.Front Cardiovasc Med. 2024 Feb 23;10:1331142. doi: 10.3389/fcvm.2023.1331142. eCollection 2023. Front Cardiovasc Med. 2024. PMID: 38463423 Free PMC article. Review.
-
Drug-coated balloon angioplasty for failing haemodialysis access: meta-analysis of randomized clinical trials.Br J Surg. 2021 Nov 11;108(11):1293-1303. doi: 10.1093/bjs/znab301. Br J Surg. 2021. PMID: 34595522 Free PMC article.
References
-
- Gerhard-Herman MD, Gornik HL, Barrett C, Barshes NR, Corriere MA, Drachman DE, Fleisher LA, Fowkes FG, Hamburg NM, Kinlay S, et al. 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2017;135:e726–e779. doi: 10.1161/CIR.0000000000000471. - PMC - PubMed
-
- Wilson S, Gelfand D, Jimenez J, Gordon I. Comparison of the results of percutaneous transluminal angioplasty and stenting with medical treatment for claudicants who have superficial femoral artery occlusive disease. Vascular. 2006;14:81–87. doi: 10.2310/6670.2006.00017. - PubMed
-
- Jongsma H, Bekken JA, de Vries JP, Verhagen HJ, Fioole B. Drug-eluting balloon angioplasty versus uncoated balloon angioplasty in patients with femoropopliteal arterial occlusive disease. J Vasc Surg. 2016;64:1503–1514. doi: 10.1016/j.jvs.2016.05.084. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

