Lung Purinoceptor Activation Triggers Ventilator-Induced Brain Injury
- PMID: 31567350
- PMCID: PMC6798751
- DOI: 10.1097/CCM.0000000000003977
Lung Purinoceptor Activation Triggers Ventilator-Induced Brain Injury
Abstract
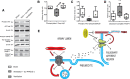
Objectives: Mechanical ventilation can cause ventilator-induced brain injury via afferent vagal signaling and hippocampal neurotransmitter imbalances. The triggering mechanisms for vagal signaling during mechanical ventilation are unknown. The objective of this study was to assess whether pulmonary transient receptor potential vanilloid type-4 (TRPV4) mechanoreceptors and vagal afferent purinergic receptors (P2X) act as triggers of ventilator-induced brain injury.
Design: Controlled, human in vitro and ex vivo studies, as well as murine in vivo laboratory studies.
Setting: Research laboratory.
Subjects: Wild-type, TRPV4-deficient C57BL/6J mice, 8-10 weeks old. Human postmortem lung tissue and human lung epithelial cell line BEAS-2B.
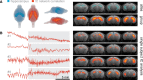
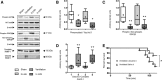
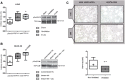
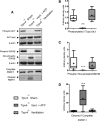
Intervention: Mice subjected to mechanical ventilation were studied using functional MRI to assess hippocampal activity. The effects of lidocaine (a nonselective ion-channel inhibitor), P2X-purinoceptor antagonist (iso-PPADS), or genetic TRPV4 deficiency on hippocampal dopamine-dependent pro-apoptotic signaling were studied in mechanically ventilated mice. Human lung epithelial cells (BEAS-2B) were used to study the effects of mechanical stretch on TRPV4 and P2X expression and activation. TRPV4 levels were measured in postmortem lung tissue from ventilated and nonventilated patients.
Measurements and main results: Hippocampus functional MRI analysis revealed considerable changes in response to the increase in tidal volume during mechanical ventilation. Intratracheal lidocaine, iso-PPADS, and TRPV4 genetic deficiency protected mice against ventilationinduced hippocampal pro-apoptotic signaling. Mechanical stretch in both, BEAS-2B cells and ventilated wild-type mice, resulted in TRPV4 activation and reduced Trpv4 and P2x expression. Intratracheal replenishment of adenosine triphosphate in Trpv4 mice abrogated the protective effect of TRPV4 deficiency. Autopsy lung tissue from ventilated patients showed decreased lung TRPV4 levels compared with nonventilated CONCLUSIONS:: TRPV4 mechanosensors and purinergic receptors are involved in the mechanisms of ventilator-induced brain injury. Inhibition of this neural signaling, either using nonspecific or specific inhibitors targeting the TRPV4/adenosine triphosphate/P2X signaling axis, may represent a novel strategy to prevent or treat ventilator-induced brain injury.
Figures
References
-
- dos Santos CC, Shan Y, Akram A, et al. Neuroimmune regulation of ventilator-induced lung injury. Am J Respir Crit Care Med 2011; 183:471–482 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

