Human herpesvirus 6 in transplant recipients: an update on diagnostic and treatment strategies
- PMID: 31567413
- PMCID: PMC7141773
- DOI: 10.1097/QCO.0000000000000592
Human herpesvirus 6 in transplant recipients: an update on diagnostic and treatment strategies
Abstract
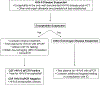
Purpose of review: The current review article focuses on recent advances in the approach to the diagnosis and treatment of human herpesvirus 6B (HHV-6B) in hematopoietic cell and solid organ transplant recipients.
Recent findings: Over the past few years, key studies have broadened our understanding of best practices for the prevention and treatment of HHV-6B encephalitis after transplantation. Moreover, important data have been reported that support a potential role of HHV-6B reactivation in the development of acute graft-versus-host disease and lower respiratory tract disease in transplant recipients. Finally, increasing recognition of inherited chromosomally integrated HHV-6 (iciHHV-6) and an expanding array of diagnostic tools have increased our understanding of the potential for complications related to viral reactivation originating from iciHHV-6 in donors or recipients.
Summary: Recent advances in diagnostic tools, disease associations, and potential treatments for HHV-6B present abundant opportunities for improving our understanding and management of this complex virus in transplant recipients.
Conflict of interest statement
Conflicts of interest:
J.A.H. has served as a consultant for Nohla Therapeutics, Inc. and Amplyx and has received research support from Nohla Therapeutics, Karius, and Takeda (formerly Shire), all unrelated to the material in this publication.
Figures

References
-
- Zerr DM, Meier AS, Selke SS, Frenkel LM, Huang M-L, Wald A, Rhoads MP, Nguy L, Bornemann R, Morrow RA, et al.: A population-based study of primary human herpesvirus 6 infection. N Engl J Med 2005, 352:768–76. - PubMed
-
-
Pellett Madan R, Hand J: Human herpesvirus 6, 7, and 8 in solid organ transplantation: Guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transplant 2019, doi:10.1111/ctr.13518. ••Updated guidelines for the management of HHV-6 after solid organ transplantation.
-
-
-
Hill JA, Mayer BT, Xie H, Leisenring WM, Huang M-L, Stevens-Ayers T, Milano F, Delaney C, Sorror ML, Sandmaier BM, et al.: The cumulative burden of double-stranded DNA virus detection after allogeneic HCT is associated with increased mortality. Blood 2017, 129. •Demonstration of the pattern of HHV-6B reactivation after multiple types of allogeneic HCT regimens.
-
-
-
Huang Y-T, Kim SJ, Lee YJ, Burack D, Nichols P, Maloy M, Perales M-A, Giralt SA, Jakubowski AA, Papanicolaou GA: Co-Infections by Double-Stranded DNA Viruses after Ex Vivo T Cell–Depleted, CD34+ Selected Hematopoietic Cell Transplantation. Biol Blood Marrow Transplant 2017, 23:1759–1766. •Demonstration of the pattern of HHV-6B reactivation after T cell-depleted allogeneic HCT.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

