The efficacy and safety of long-term add-on treatment of azithromycin in asthma: A systematic review and meta-analysis
- PMID: 31567962
- PMCID: PMC6756741
- DOI: 10.1097/MD.0000000000017190
The efficacy and safety of long-term add-on treatment of azithromycin in asthma: A systematic review and meta-analysis
Abstract
Aim: Effects of azithromycin on asthma reported in clinical trials are less consistent. We aimed to further clarify the efficacy and safety of azithromycin in treatment of asthma.
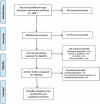
Methods: The protocol registration number was CRD42017074318 (http://www.crd.york.ac.uk/Prospero). We searched PubMed, EMBASE, Cochrane databases, China National Knowledge Internet (CNKI), and Wanfang databases for the randomized controlled trials (RCTs) with prolonged treatment of azithromycin for more than 3 weeks. Random-effects or fixed-effects model was applied to calculate risk ratio (RR) and mean difference (MD) for dichotomous and continuous data respectively.
Results: A total of eight studies were included for analysis. The pooled result of adjunctive azithromycin therapy in asthma showed a small, but statistically significant increase in forced expiratory volume in one second (FEV1) (MD = 0.06, 95% confidence interval [CI]: 0.01-0.12, P = .02), but no significant differences in exacerbation frequency (MD = -0.42, 95%CI: -1.13 to 0.30, P = .25) and peak expiratory flow (PEF) (MD = 0.20, 95% CI: -0.05 to 0.44, P = .12), fractional exhaled nitric oxide (FeNO) (MD = 4.12, 95% CI: -2.06 to 10.30, P = .19), asthma quality of life questionnaire (AQLQ) (MD: 0.05, 95% CI: -0.17 to 0.28, P = .65), asthma control questionnaire (ACQ) (MD: -0.03, 95% CI: -0.21 to 0.15, P = .75). The subgroup analysis revealed that azithromycin could decrease FeNO among Asian asthma (MD = 15.04, 95% CI: 6.18-23.90, P = .0009).
Conclusions: Add-on therapy of azithromycin in asthma patients could improve the FEV1, but failed to improve asthma exacerbations, PEF, ACQ, AQLQ, and FeNO. Subgroup analysis indicated that azithromycin could improve FeNO in Asian group asthmatics.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Competing interests: The authors declare that they have no competing interests.
Figures





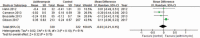


References
-
- Weiss KB, Sullivan SD. The health economics of asthma and rhinitis. I. Assessing the economic impact. J Allergy Clin Immunol 2001;107:3–8. - PubMed
-
- Calhoun WJ, Haselkorn T, Mink DR, et al. Clinical burden and predictors of asthma exacerbations in patients on guideline-based steps 4–6 asthma therapy in the TENOR cohort. J Allergy Clin Immunol Pract 2014;2:193–200. - PubMed
-
- Castro M, Zangrilli J, Wechsler ME, et al. Reslizumab for inadequately controlled asthma with elevated blood eosinophil counts: results from two multicentre, parallel, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet Respir Med 2015;3:355–66. - PubMed
-
- Ortega HG, Liu MC, Pavord ID, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med 2014;371:1198–207. - PubMed
-
- Parnham MJ, Erakovic Haber V, Giamarellos-Bourboulis EJ, et al. Azithromycin: mechanisms of action and their relevance for clinical applications. Pharmacol Ther 2014;143:225–45. - PubMed

