Pyruvate produced by Brugia spp. via glycolysis is essential for maintaining the mutualistic association between the parasite and its endosymbiont, Wolbachia
- PMID: 31568486
- PMCID: PMC6791551
- DOI: 10.1371/journal.ppat.1008085
Pyruvate produced by Brugia spp. via glycolysis is essential for maintaining the mutualistic association between the parasite and its endosymbiont, Wolbachia
Abstract
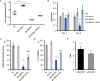
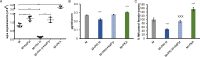
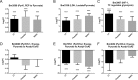
Human parasitic nematodes are the causative agents of lymphatic filariasis (elephantiasis) and onchocerciasis (river blindness), diseases that are endemic to more than 80 countries and that consistently rank in the top ten for the highest number of years lived with disability. These filarial nematodes have evolved an obligate mutualistic association with an intracellular bacterium, Wolbachia, a symbiont that is essential for the successful development, reproduction, and survival of adult filarial worms. Elimination of the bacteria causes adult worms to die, making Wolbachia a primary target for developing new interventional tools to combat filariases. To further explore Wolbachia as a promising indirect macrofilaricidal drug target, the essential cellular processes that define the symbiotic Wolbachia-host interactions need to be identified. Genomic analyses revealed that while filarial nematodes encode all the enzymes necessary for glycolysis, Wolbachia does not encode the genes for three glycolytic enzymes: hexokinase, 6-phosphofructokinase, and pyruvate kinase. These enzymes are necessary for converting glucose into pyruvate. Wolbachia, however, has the full complement of genes required for gluconeogenesis starting with pyruvate, and for energy metabolism via the tricarboxylic acid cycle. Therefore, we hypothesized that Wolbachia might depend on host glycolysis to maintain a mutualistic association with their parasitic host. We did conditional experiments in vitro that confirmed that glycolysis and its end-product, pyruvate, sustain this symbiotic relationship. Analysis of alternative sources of pyruvate within the worm indicated that the filarial lactate dehydrogenase could also regulate the local intracellular concentration of pyruvate in proximity to Wolbachia and thus help control bacterial growth via molecular interactions with the bacteria. Lastly, we have shown that the parasite's pyruvate kinase, the enzyme that performs the last step in glycolysis, could be a potential novel anti-filarial drug target. Establishing that glycolysis is an essential component of symbiosis in filarial worms could have a broader impact on research focused on other intracellular bacteria-host interactions where the role of glycolysis in supporting intracellular survival of bacteria has been reported.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

