Letrozole treatment of pubertal female mice results in activational effects on reproduction, metabolism and the gut microbiome
- PMID: 31568518
- PMCID: PMC6768472
- DOI: 10.1371/journal.pone.0223274
Letrozole treatment of pubertal female mice results in activational effects on reproduction, metabolism and the gut microbiome
Abstract
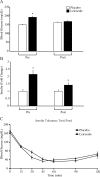
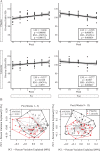
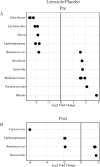
Polycystic ovary syndrome (PCOS) is a common endocrine disorder in reproductive-aged women that is comprised of two out of the following three features: hyperandrogenism, oligo- or amenorrhea, or polycystic ovaries. In addition to infertility, many women with PCOS have metabolic dysregulation that increases the risk of developing type 2 diabetes, hypertension, and non-alcoholic fatty liver disease. Changes in the gut microbiome are associated with PCOS and gut microbes may be involved in the pathology of this disorder. Since PCOS often manifests in the early reproductive years, puberty is considered to be a critical time period for the development of PCOS. Exposure to sex steroid hormones during development results in permanent, organizational effects, while activational effects are transient and require the continued presence of the hormone. Androgens exert organizational effects during prenatal or early post-natal development, but it is unclear whether androgen excess results in organizational or activational effects during puberty. We recently developed a letrozole-induced PCOS mouse model that recapitulates both reproductive and metabolic phenotypes of PCOS. In this study, we investigated whether letrozole treatment of pubertal female mice exerts organizational or activational effects on host physiology and the gut microbiome. Two months after letrozole removal, we observed recovery of reproductive and metabolic parameters, as well as diversity and composition of the gut microbiome, indicating that letrozole treatment of female mice during puberty resulted in predominantly activational effects. These results suggest that if exposure to excess androgens during puberty leads to the development of PCOS, reduction of androgen levels during this time may improve reproductive and metabolic phenotypes in women with PCOS. These results also imply that continuous letrozole exposure is required to model PCOS in pubertal female mice since letrozole exerts activational rather than organizational effects during puberty.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures