Exploring the Trans-Cleavage Activity of CRISPR-Cas12a (cpf1) for the Development of a Universal Electrochemical Biosensor
- PMID: 31568601
- PMCID: PMC6938695
- DOI: 10.1002/anie.201910772
Exploring the Trans-Cleavage Activity of CRISPR-Cas12a (cpf1) for the Development of a Universal Electrochemical Biosensor
Abstract
An accurate, rapid, and cost-effective biosensor for the quantification of disease biomarkers is vital for the development of early-diagnostic point-of-care systems. The recent discovery of the trans-cleavage property of CRISPR type V effectors makes CRISPR a potential high-accuracy bio-recognition tool. Herein, a CRISPR-Cas12a (cpf1) based electrochemical biosensor (E-CRISPR) is reported, which is more cost-effective and portable than optical-transduction-based biosensors. Through optimizing the in vitro trans-cleavage activity of Cas12a, E-CRIPSR was used to detect viral nucleic acids, including human papillomavirus 16 (HPV-16) and parvovirus B19 (PB-19), with a picomolar sensitivity. An aptamer-based E-CRISPR cascade was further designed for the detection of transforming growth factor β1 (TGF-β1) protein in clinical samples. As demonstrated, E-CRISPR could enable the development of portable, accurate, and cost-effective point-of-care diagnostic systems.
Keywords: CRISPR Cas12a (cpf1); bioanalytical chemistry; biosensor; electrochemistry; trans-acting cleavage.
© 2019 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
Conflict of interest statement
Conflict of interest
The authors declare no conflict of interest.
Figures
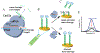


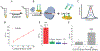
Similar articles
-
Amplification-Free CRISPR/Cas12a-Based Electrochemical Biosensor with Enhanced Sensitivity for Viral Detection.ACS Sens. 2025 Jun 27;10(6):4361-4370. doi: 10.1021/acssensors.5c00576. Epub 2025 May 22. ACS Sens. 2025. PMID: 40403178
-
CRISPR/Cas12a-based electrochemical biosensor for highly sensitive detection of cTnI.Bioelectrochemistry. 2022 Aug;146:108167. doi: 10.1016/j.bioelechem.2022.108167. Epub 2022 May 19. Bioelectrochemistry. 2022. PMID: 35623274
-
CRISPR/Cas12a Powered DNA Framework-Supported Electrochemical Biosensing Platform for Ultrasensitive Nucleic Acid Analysis.Small Methods. 2021 Dec;5(12):e2100935. doi: 10.1002/smtd.202100935. Epub 2021 Oct 10. Small Methods. 2021. PMID: 34928030
-
Towards application of CRISPR-Cas12a in the design of modern viral DNA detection tools (Review).J Nanobiotechnology. 2022 Jan 21;20(1):41. doi: 10.1186/s12951-022-01246-7. J Nanobiotechnology. 2022. PMID: 35062978 Free PMC article. Review.
-
Recent progress in aptamer and CRISPR-Cas12a based systems for non-nucleic target detection.Crit Rev Anal Chem. 2024;54(7):2670-2687. doi: 10.1080/10408347.2023.2197062. Epub 2023 Apr 8. Crit Rev Anal Chem. 2024. PMID: 37029907 Review.
Cited by
-
Point-of-care CRISPR/Cas nucleic acid detection: Recent advances, challenges and opportunities.Biosens Bioelectron. 2020 Oct 15;166:112445. doi: 10.1016/j.bios.2020.112445. Epub 2020 Jul 26. Biosens Bioelectron. 2020. PMID: 32758911 Free PMC article. Review.
-
An ultrasensitive hybridization chain reaction-amplified CRISPR-Cas12a aptasensor for extracellular vesicle surface protein quantification.Theranostics. 2020 Aug 13;10(22):10262-10273. doi: 10.7150/thno.49047. eCollection 2020. Theranostics. 2020. PMID: 32929347 Free PMC article.
-
Coupling nucleic acid circuitry with the CRISPR-Cas12a system for universal and signal-on detection.RSC Adv. 2022 Apr 4;12(17):10374-10378. doi: 10.1039/d2ra01332k. eCollection 2022 Mar 31. RSC Adv. 2022. PMID: 35425009 Free PMC article.
-
CRISPR-Cas12a-mediated label-free electrochemical aptamer-based sensor for SARS-CoV-2 antigen detection.Bioelectrochemistry. 2022 Aug;146:108105. doi: 10.1016/j.bioelechem.2022.108105. Epub 2022 Mar 19. Bioelectrochemistry. 2022. PMID: 35367933 Free PMC article.
-
Identifying Selectivity Filters in Protein Biosensor for Ligand Screening.JACS Au. 2023 Sep 18;3(10):2800-2812. doi: 10.1021/jacsau.3c00374. eCollection 2023 Oct 23. JACS Au. 2023. PMID: 37885591 Free PMC article.
References
-
- Kelley SO, Mirkin CA, Walt DR, Ismagilov RF, Toner M, Sargent EH, Nature nanotechnology 2014, 9, 969; - PMC - PubMed
- Yang Y, Gao W, Chemical Society Reviews 2019, 48, 1465–1491; - PubMed
- Kim J, Campbell AS, de Ávila BE-F, Wang J, Nature Biotechnology 2019, 37, 389–406; - PMC - PubMed
- Dai Y, Liu CC, Angewandte Chemie International Edition 2019, 58, 12355–12368; - PubMed
- Wu Y, Tilley RD, Gooding JJ, Journal of the American Chemical Society 2019, 141, 1162–1170; - PubMed
- Furst AL, Francis MB, Chemical Reviews 2019, 119, 700–726; - PubMed
- Zwang TJ, Tse ECM, Barton JK, ACS Chemical Biology 2018, 13, 1799–1809; - PMC - PubMed
- Lubin AA, Plaxco KW, Accounts of Chemical Research 2010, 43, 496–505; - PMC - PubMed
- Yang F, Li Q, Wang L, Zhang G-J, Fan C, ACS Sensors 2018, 3, 903–919. - PubMed
-
- Gootenberg JS, Abudayyeh OO, Lee JW, Essletzbichler P, Dy AJ, Joung J, Verdine V, Donghia N, Daringer NM, Freije CA, Science 2017, 356, 438–442; - PMC - PubMed
- Li Y, Li S, Wang J, Liu G, Trends Biotechnol 2019, 37, 730–743; - PubMed
- Leung K, Krishnan Y, ACS Central Science 2019, 5, 1111–1113; - PMC - PubMed
- Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N, Hsu PD, Wu X, Jiang W, Marraffini LA, Science 2013, 339, 819–823; - PMC - PubMed
- Huang TP, Zhao KT, Miller SM, Gaudelli NM, Oakes BL, Fellmann C, Savage DF, Liu DR, Nature Biotechnology 2019, 37, 626–631; - PMC - PubMed
- Hu JH, Miller SM, Geurts MH, Tang W, Chen L, Sun N, Zeina CM, Gao X, Rees HA, Lin Z, Liu DR, Nature 2018, 556, 57; - PMC - PubMed
- Yin H, Song C-Q, Suresh S, Kwan S-Y, Wu Q, Walsh S, Ding J, Bogorad RL, Zhu LJ, Wolfe SA, Koteliansky V, Xue W, Langer R, Anderson DG, Nature Chemical Biology 2018, 14, 311. - PMC - PubMed
-
- Chen JS, Ma E, Harrington LB, Da Costa M, Tian X, Palefsky JM, Doudna JA, Science 2018, 360, 436–439; - PMC - PubMed
- Gootenberg JS, Abudayyeh OO, Kellner MJ, Joung J, Collins JJ, Zhang F, Science 2018, 360, 439–444; - PMC - PubMed
- Li S-Y, Cheng Q-X, Wang J-M, Li X-Y, Zhang Z-L, Gao S, Cao R-B, Zhao G-P, Wang J, Cell Discovery 2018, 4, 20; - PMC - PubMed
- Li S-Y, Cheng Q-X, Liu J-K, Nie X-Q, Zhao G-P, Wang J, Cell research 2018, 28, 491. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

