Adherence to pan-genotypic glecaprevir/pibrentasvir and efficacy in HCV-infected patients: A pooled analysis of clinical trials
- PMID: 31568620
- PMCID: PMC7187170
- DOI: 10.1111/liv.14266
Adherence to pan-genotypic glecaprevir/pibrentasvir and efficacy in HCV-infected patients: A pooled analysis of clinical trials
Abstract
Background & aims: Adequate adherence to hepatitis C virus (HCV) treatment is believed to be a key component of treatment success because non-adherence can potentially result in treatment failure and the emergence of resistant viral variants. This analysis assessed factors associated with non-adherence to glecaprevir/pibrentasvir (G/P) therapy and the impact of non-adherence on sustained virological response at post-treatment week 12 (SVR12) rates in HCV genotype (GT) 1-6-infected patients.
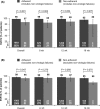
Methods: Adherence was calculated by pill counts at study visits during treatment, and defined as having a lowest treatment adherence of ≥80% and ≤120% at each study visit. Exploratory logistic regression modelling assessed predictors of non-adherence to G/P therapy. SVR12 rates by treatment adherence were assessed in the intent-to-treat (ITT) population and modified ITT (mITT) population, which excludes non-virological failures.
Results: Overall, 97% (2024/2091) of patients were adherent to G/P therapy at all consecutive study visits. Alcohol use was the only baseline characteristic independently associated with non-adherence to G/P therapy (OR: 2.38; 95% CI: 1.13-5.01; P = .022). In the mITT population, overall SVR12 rates were high both in patients who were adherent to G/P therapy and those who were not (99% [1983/2008] and 95% [58/61] respectively; P = .047). Corresponding SVR12 rates in the ITT population were 98% (1983/2024) and 87% (58/67) respectively.
Conclusions: Most patients adhered to G/P therapy. SVR12 rates were high both in patients who were adherent to G/P treatment and those who were not. Patient education on treatment adherence should remain an important part of HCV treatment.
Clinical trials registration: NCT02604017, NCT02640482, NCT02640157, NCT02636595, NCT02642432, NCT02651194, NCT02243293, NCT02446717.
Keywords: G/P; adherence; glecaprevir; hepatitis C virus; pibrentasvir.
© 2019 The Authors. Liver International published by John Wiley & Sons Ltd.
Conflict of interest statement
A Brown: Advisor and speaker for, and recipient of research grants from, AbbVie, Bristol‐Meyers Squibb, Janssen, Gilead Sciences and MSD. TM Welzel: Consultant or speaker for AbbVie, Boehringer Ingelheim, Bristol‐Myers Squibb, Gilead Sciences and Janssen. B Conway: Research and grant support from, and participation in advisory boards for, AbbVie, Bristol‐Myers Squibb, Gilead Sciences, Janssen and Merck. F Negro: Grant support from Gilead Sciences; advisor for AbbVie, Gilead Sciences and Merck. N Bräu: Advisor and speaker for, and received grant support from, AbbVie, Bristol‐Myers Squibb, Gilead Sciences and Merck. J Grebely: Consultant/advisor for, or received grant support from, AbbVie, Cepheid, Gilead Sciences, and Merck/MSD. M Puoti: Temporary advisory board and/or speaker at own events for AbbVie, BMS, Boehringer Ingelheim, Janssen, Gilead Sciences, MSD and Roche; research support from Gilead Sciences and MSD. A Aghemo: Grant support from Gilead Sciences and AbbVie; advisory board and speaker for AbbVie, BMS, Gilead Sciences, Janssen and MSD. H Kleine, D Pugatch, FJ Mensa, YJ Chen, Y Lei: current or former employees of AbbVie, Inc; may own AbbVie stock and/or options. E Lawitz: Consultant, advisor and speaker for, or received research/grant support from AbbVie and Gilead Sciences, T Asselah: Clinical investigator, speaker and consultant for AbbVie, Gilead Sciences, Janssen Pharmaceuticals, Merck Sharp & Dohme, and Roche.
Figures
References
-
- World Health Organization . Global health sector strategy on viral hepatitis 2016–2021: towards ending viral hepatitis. http://apps.who.int/iris/bitstream/10665/246177/1/WHO-HIV-2016.06-eng.pd.... Accessed April 12, 2018.
-
- Dore GJ, Feld JJ. Hepatitis C virus therapeutic development: in pursuit of "perfectovir". Clin Infect Dis. 2015;60:1829‐1836. - PubMed
-
- Pawlotsky JM. Hepatitis C drugs: is next generation the last generation? Gastroenterology. 2016;151:587‐590. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

