Non-lethal proteasome inhibition activates pro-tumorigenic pathways in multiple myeloma cells
- PMID: 31568628
- PMCID: PMC6850931
- DOI: 10.1111/jcmm.14653
Non-lethal proteasome inhibition activates pro-tumorigenic pathways in multiple myeloma cells
Abstract
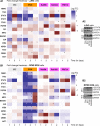
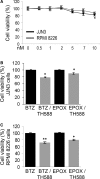
Multiple myeloma (MM) is a haematological malignancy being characterized by clonal plasma cell proliferation in the bone marrow. Targeting the proteasome with specific inhibitors (PIs) has been proven a promising therapeutic strategy and PIs have been approved for the treatment of MM and mantle-cell lymphoma; yet, while outcome has improved, most patients inevitably relapse. As relapse refers to MM cells that survive therapy, we sought to identify the molecular responses induced in MM cells after non-lethal proteasome inhibition. By using bortezomib (BTZ), epoxomicin (EPOX; a carfilzomib-like PI) and three PIs, namely Rub999, PR671A and Rub1024 that target each of the three proteasome peptidases, we found that only BTZ and EPOX are toxic in MM cells at low concentrations. Phosphoproteomic profiling after treatment of MM cells with non-lethal (IC10 ) doses of the PIs revealed inhibitor- and cell type-specific readouts, being marked by the activation of tumorigenic STAT3 and STAT6. Consistently, cytokine/chemokine profiling revealed the increased secretion of immunosuppressive pro-tumorigenic cytokines (IL6 and IL8), along with the inhibition of potent T cell chemoattractant chemokines (CXCL10). These findings indicate that MM cells that survive treatment with therapeutic PIs shape a pro-tumorigenic immunosuppressive cellular and secretory bone marrow microenvironment that enables malignancy to relapse.
Keywords: cytokines; kinases; multiple myeloma; proteasome; proteasome inhibitors.
© 2019 The Authors. Journal of Cellular and Molecular Medicine published by John Wiley & Sons Ltd and Foundation for Cellular and Molecular Medicine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Trougakos IP, Sesti F, Tsakiri E, Gorgoulis VG. Non‐enzymatic post‐translational protein modifications and proteostasis network deregulation in carcinogenesis. J Proteomics. 2013;92:274‐298. - PubMed
-
- Tsakiri EN, Trougakos IP. The amazing ubiquitin‐proteasome system: structural components and implication in aging. Int Rev Cell Mol Biol. 2015;314:171‐237. - PubMed
-
- Vilchez D, Saez I, Dillin A. The role of protein clearance mechanisms in organismal ageing and age‐related diseases. Nat Commun. 2014;5:5659. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

