Novel UL97 drug resistance mutations identified at baseline in a clinical trial of maribavir for resistant or refractory cytomegalovirus infection
- PMID: 31568799
- PMCID: PMC6892599
- DOI: 10.1016/j.antiviral.2019.104616
Novel UL97 drug resistance mutations identified at baseline in a clinical trial of maribavir for resistant or refractory cytomegalovirus infection
Abstract
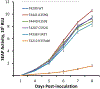
In a Phase 2 clinical trial, 120 subjects with cytomegalovirus (CMV) infection refractory or resistant to standard therapy were randomized equally to 3 doses of oral maribavir treatment, and 70% achieved undetectable plasma CMV DNA within 12 weeks. At study entry, standard diagnostic UL97 genotyping was available for 71 subjects, with 60 (85%) revealing well-characterized ganciclovir resistance mutations that did not preclude a therapeutic response to maribavir. Central laboratory testing of a range of UL97 codons (288-468) not fully covered by standard genotyping was done on 93 subjects at baseline. This detected no previously known maribavir resistance mutations, but identified atypical mutations in 3 subjects, including a P-loop substitution F342Y, and ATP-binding region substitutions K359E/Q. By recombinant phenotyping, K359E and K359Q each conferred a nearly 4-fold increased ganciclovir 50% inhibitory concentration (EC50) without maribavir resistance, whereas F342Y conferred a 6-fold increased ganciclovir EC50 and a 4.5-fold increased maribavir EC50. The subject with F342Y detected at baseline did not achieve plasma CMV DNA clearance after 12 weeks of maribavir therapy and later developed an additional UL97 substitution H411Y known to confer 12- to 20-fold increased MBV EC50 by itself. The combination of F342Y and H411Y was shown to increase the maribavir EC50 by 56-fold. Diagnostic genotyping of UL97 should be expanded to cover the ATP-binding region beginning at codon 335 to enable the detection of atypical resistance mutations and further correlation of their clinical significance.
Keywords: Antiviral drug resistance; Cross-resistance; Cytomegalovirus; Ganciclovir; Maribavir.
Copyright © 2019 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Competing interests: SC is principal investigator of Collaborative Research and Development agreements between the Department of Veterans Affairs (VA) and Merck for recombinant phenotyping. JW, KS, and TB are employees of, and hold stock/stock options in, Shire.
Figures
References
-
- Drew WL, Miner RC, Marousek GI, Chou S, 2006. Maribavir sensitivity of cytomegalovirus isolates resistant to ganciclovir, cidofovir or foscarnet. J Clin Virol 37, 124–127. - PubMed

