Role of Memory B Cells in Hemagglutinin-Specific Antibody Production Following Human Influenza A Virus Infection
- PMID: 31569328
- PMCID: PMC6963758
- DOI: 10.3390/pathogens8040167
Role of Memory B Cells in Hemagglutinin-Specific Antibody Production Following Human Influenza A Virus Infection
Abstract
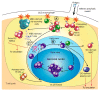
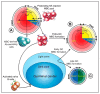
When influenza A virus infects an immune individual, preexisting memory B cell (MBC) activation and rapid anamnestic antibody production plays a key role in viral clearance. The most effective neutralizing antibodies target the antigenically variable head of the viral hemagglutinin (HA); antibodies against the conserved HA stalk provide broader but less potent protection. In this review, we provide a comprehensive picture of an adult's HA-specific antibody response to influenza virus infection. The process is followed from preexisting HA-specific MBC activation and rapid production of anti-HA antibodies, through to germinal center seeding and adaptation of the response to novel features of the HA. A major focus of the review is the role of competition between preexisting MBCs in determining the character of the HA-reactive antibody response. HA novelty modifies this competition and can shift the response from the immunodominant head to the stalk. We suggest that antibodies resulting from preexisting MBC activation are important regulators of anti-HA antibody production and play a role in positive selection of germinal center B cells reactive to novel HA epitopes. Our review also considers the role of MBCs in the effects of early-life imprinting on HA head- and stalk-specific antibody responses to influenza infection. An understanding of the processes described in this review will guide development of vaccination strategies that provide broadly effective protection.
Keywords: antibodies; germinal centers; hemagglutin stalk; hemagglutinin; imprinting; infection; influenza A virus; memory B cells; original antigenic sin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

