FADD in Cancer: Mechanisms of Altered Expression and Function, and Clinical Implications
- PMID: 31569512
- PMCID: PMC6826683
- DOI: 10.3390/cancers11101462
FADD in Cancer: Mechanisms of Altered Expression and Function, and Clinical Implications
Abstract
FADD was initially described as an adaptor molecule for death receptor-mediated apoptosis, but subsequently it has been implicated in nonapoptotic cellular processes such as proliferation and cell cycle control. During the last decade, FADD has been shown to play a pivotal role in most of the signalosome complexes, such as the necroptosome and the inflammasome. Interestingly, various mechanisms involved in regulating FADD functions have been identified, essentially posttranslational modifications and secretion. All these aspects have been thoroughly addressed in previous reviews. However, FADD implication in cancer is complex, due to pleiotropic effects. It has been reported either as anti- or protumorigenic, depending on the cell type. Regulation of FADD expression in cancer is a complex issue since both overexpression and downregulation have been reported, but the mechanisms underlying such alterations have not been fully unveiled. Posttranslational modifications also constitute a relevant mechanism controlling FADD levels and functions in tumor cells. In this review, we aim to provide detailed, updated information on alterations leading to changes in FADD expression and function in cancer. The participation of FADD in various biological processes is recapitulated, with a mention of interesting novel functions recently proposed for FADD, such as regulation of gene expression and control of metabolic pathways. Finally, we gather all the available evidence regarding the clinical implications of FADD alterations in cancer, especially as it has been proposed as a potential biomarker with prognostic value.
Keywords: FADD; chromosomal alterations; clinical implications; epigenetic regulation; gene expression; metabolism; mutations; polymorphisms; posttranslational modifications; transcription factors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





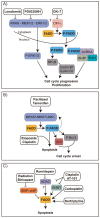
Similar articles
-
FADD at the Crossroads between Cancer and Inflammation.Trends Immunol. 2018 Dec;39(12):1036-1053. doi: 10.1016/j.it.2018.10.005. Epub 2018 Nov 3. Trends Immunol. 2018. PMID: 30401514 Review.
-
FADD adaptor in cancer.Med Immunol. 2005 Feb 17;4(1):1. doi: 10.1186/1476-9433-4-1. Med Immunol. 2005. PMID: 15717929 Free PMC article.
-
Functional specific roles of FADD: comparative proteomic analyses from knockout cell lines.Mol Biosyst. 2013 Aug;9(8):2063-78. doi: 10.1039/c3mb70023b. Epub 2013 May 21. Mol Biosyst. 2013. PMID: 23689606
-
The classical NLRP3 inflammasome controls FADD unconventional secretion through microvesicle shedding.Cell Death Dis. 2019 Feb 25;10(3):190. doi: 10.1038/s41419-019-1412-9. Cell Death Dis. 2019. PMID: 30804327 Free PMC article.
-
FADD and its phosphorylation.IUBMB Life. 2004 Jul;56(7):395-401. doi: 10.1080/15216540400008929. IUBMB Life. 2004. PMID: 15545216 Review.
Cited by
-
Prognostic and Clinicopathological Significance of FADD Upregulation in Head and Neck Squamous Cell Carcinoma: A Systematic Review and Meta-Analysis.Cancers (Basel). 2020 Aug 24;12(9):2393. doi: 10.3390/cancers12092393. Cancers (Basel). 2020. PMID: 32847023 Free PMC article. Review.
-
BCL2A1 regulates Canady Helios Cold Plasma-induced cell death in triple-negative breast cancer.Sci Rep. 2022 Mar 8;12(1):4038. doi: 10.1038/s41598-022-07027-4. Sci Rep. 2022. PMID: 35260587 Free PMC article.
-
Identification and analysis of prognostic immune cell homeostasis characteristics in lung adenocarcinoma.Clin Respir J. 2024 May;18(5):e13755. doi: 10.1111/crj.13755. Clin Respir J. 2024. PMID: 38757752 Free PMC article.
-
Proliferation and Apoptosis Pathways and Factors in Oral Squamous Cell Carcinoma.Int J Mol Sci. 2022 Jan 29;23(3):1562. doi: 10.3390/ijms23031562. Int J Mol Sci. 2022. PMID: 35163485 Free PMC article. Review.
-
Metabolic genes, a potential predictor of prognosis and immunogenicity of clear cell renal cell carcinoma.Am J Cancer Res. 2023 Apr 15;13(4):1387-1406. eCollection 2023. Am J Cancer Res. 2023. PMID: 37168330 Free PMC article.
References
-
- Yeh W.C., de la Pompa J.L., McCurrach M.E., Shu H.B., Elia A.J., Shahinian A., Ng M., Wakeham A., Khoo W., Mitchell K., et al. FADD: Essential for embryo development and signaling from some, but not all, inducers of apoptosis. Science. 1998;279:1954–1958. doi: 10.1126/science.279.5358.1954. - DOI - PubMed
-
- Fagerberg L., Hallstrom B.M., Oksvold P., Kampf C., Djureinovic D., Odeberg J., Habuka M., Tahmasebpoor S., Danielsson A., Edlund K., et al. Analysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics. Mol. Cell. Proteom. 2014;13:397–406. doi: 10.1074/mcp.M113.035600. - DOI - PMC - PubMed
-
- Callegari C.C., Cavalli I.J., Lima R.S., Jucoski T.S., Torresan C., Urban C.A., Kuroda F., Anselmi K.F., Cavalli L.R., Ribeiro E.M. Copy number and expression analysis of FOSL1, GSTP1, NTSR1, FADD and CCND1 genes in primary breast tumors with axillary lymph node metastasis. Cancer Genet. 2016;209:331–339. doi: 10.1016/j.cancergen.2016.06.003. - DOI - PubMed
-
- Chen G., Bhojani M.S., Heaford A.C., Chang D.C., Laxman B., Thomas D.G., Griffin L.B., Yu J., Coppola J.M., Giordano T.J., et al. Phosphorylated FADD induces NF-kappaB, perturbs cell cycle, and is associated with poor outcome in lung adenocarcinomas. Proc. Natl. Acad. Sci. USA. 2005;102:12507–12512. doi: 10.1073/pnas.0500397102. - DOI - PMC - PubMed
Publication types
Grants and funding
- RTI2018-093330-B-I00/Ministerio de Economía y Competitividad
- BES-2013-065740/Ministerio de Economía y Competitividad
- Medicina de precisión y cáncer/Fundación Ramón Areces
- B2017/BMD-3778 LINFOMAS-CM/Consejería de Sanidad, Comunidad de Madrid
- AECC 2018 PROYE18054PIRI/Fundación Científica Asociación Española Contra el Cáncer

