Adipocytes and microRNAs Crosstalk: A Key Tile in the Mosaic of Breast Cancer Microenvironment
- PMID: 31569710
- PMCID: PMC6826993
- DOI: 10.3390/cancers11101451
Adipocytes and microRNAs Crosstalk: A Key Tile in the Mosaic of Breast Cancer Microenvironment
Abstract
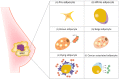
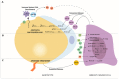
Breast cancer (BC) is a disease characterized by a high grade of heterogeneity. Consequently, despite the great achievements obtained in the last decades, most of the current therapeutic regimens still fail. The identification of new molecular mechanisms that will increase the knowledge of all steps of tumor initiation and growth is mandatory in finding new clinical strategies. The BC microenvironment, consisting of endothelial cells, fibroblasts, immune cells and adipocytes, plays an essential role in regulating BC development, and recently it has gained great attention in the scientific community. In particular, adipose tissue is emerging as an important target to investigate among mammary gland components. The mechanisms underlying BC progression driven by adipocytes are predominantly unexplored, especially that involving the switch from normal adipocytes to the so-called cancer-associated adipocytes (CAAs). MicroRNAs (miRNAs), a class of gene expression modulators, have emerged as the regulators of key oncogenes and tumor suppressor genes that affect multiple pathways of the tumor microenvironment and adipose tissue. This review concerns a presentation of the role of adipocytes in breast tissue, and describes the most recent discoveries about the interplay between adipocytes and miRNAs, which collaborate in the arrangement of a pro-inflammatory and cancerous microenvironment, laying the foundations for new concepts in the prevention and treatment of BC.
Keywords: Breast Cancer; adipocytes; microRNAs.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Vu L.T., Peng B., Zhang D.X., Ma V., Mathey-Andrews C.A., Lam C.K., Kiomourtzis T., Jin J., McReynolds L., Huang L., et al. Tumor-secreted extracellular vesicles promote the activation of cancer-associated fibroblasts via the transfer of microRNA-125b. J. Extracell. Vesicles. 2019;8:1599680. doi: 10.1080/20013078.2019.1599680. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

