3D Bioprinting of the Sustained Drug Release Wound Dressing with Double-Crosslinked Hyaluronic-Acid-Based Hydrogels
- PMID: 31569810
- PMCID: PMC6835267
- DOI: 10.3390/polym11101584
3D Bioprinting of the Sustained Drug Release Wound Dressing with Double-Crosslinked Hyaluronic-Acid-Based Hydrogels
Abstract
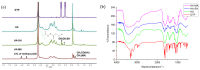
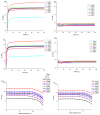
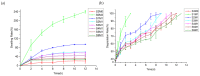
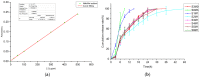
Hyaluronic acid (HA)-based hydrogels are widely used in biomedical applications due to their excellent biocompatibility. HA can be Ultraviolet (UV)-crosslinked by modification with methacrylic anhydride (HA-MA) and crosslinked by modification with 3,3'-dithiobis(propionylhydrazide) (DTP) (HA-SH) via click reaction. In the study presented in this paper, a 3D-bioprinted, double-crosslinked, hyaluronic-acid-based hydrogel for wound dressing was proposed. The hydrogel was produced by mixing HA-MA and HA-SH at different weight ratios. The rheological test showed that the storage modulus (G') of the HA-SH/HA-MA hydrogel increased with the increase in the HA-MA content. The hydrogel had a high swelling ratio and a high controlled degradation rate. The in vitro degradation test showed that the hydrogel at the HA-SH/HA-MA ratio of 9:1 (S9M1) degraded by 89.91% ± 2.26% at 11 days. The rheological performance, drug release profile and the cytocompatibility of HA-SH/HA-MA hydrogels with loaded Nafcillin, which is an antibacterial drug, were evaluated. The wound dressing function of this hydrogel was evaluated by Live/Dead staining and CCK-8 assays. The foregoing results imply that the proposed HA-SH/HA-MA hydrogel has promise in wound repair applications.
Keywords: 3D bioprinting; biodegradable; double-crosslinked; hyaluronic acid; wound dressing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








Similar articles
-
Synthesis and degradation test of hyaluronic acid hydrogels.Int J Biol Macromol. 2007 Mar 10;40(4):374-80. doi: 10.1016/j.ijbiomac.2006.09.019. Epub 2006 Oct 14. Int J Biol Macromol. 2007. PMID: 17101173
-
In situ Forming Hyperbranched PEG-Thiolated Hyaluronic Acid Hydrogels With Honey-Mimetic Antibacterial Properties.Front Bioeng Biotechnol. 2021 Nov 16;9:742135. doi: 10.3389/fbioe.2021.742135. eCollection 2021. Front Bioeng Biotechnol. 2021. PMID: 34869257 Free PMC article.
-
Heparin-hyaluronic acid hydrogel in support of cellular activities of 3D encapsulated adipose derived stem cells.Acta Biomater. 2017 Feb;49:284-295. doi: 10.1016/j.actbio.2016.12.001. Epub 2016 Dec 5. Acta Biomater. 2017. PMID: 27919839
-
Alginate-based hydrogels as drug delivery vehicles in cancer treatment and their applications in wound dressing and 3D bioprinting.J Biol Eng. 2020 Mar 13;14:8. doi: 10.1186/s13036-020-0227-7. eCollection 2020. J Biol Eng. 2020. PMID: 32190110 Free PMC article. Review.
-
From crosslinking strategies to biomedical applications of hyaluronic acid-based hydrogels: A review.Int J Biol Macromol. 2023 Mar 15;231:123308. doi: 10.1016/j.ijbiomac.2023.123308. Epub 2023 Jan 17. Int J Biol Macromol. 2023. PMID: 36669634 Review.
Cited by
-
A More Biomimetic Cell Migration Assay with High Reliability and Its Applications.Pharmaceuticals (Basel). 2022 Jun 1;15(6):695. doi: 10.3390/ph15060695. Pharmaceuticals (Basel). 2022. PMID: 35745614 Free PMC article.
-
Hydrogel Network Architecture Design Space: Impact on Mechanical and Viscoelastic Properties.Gels. 2025 Jul 30;11(8):588. doi: 10.3390/gels11080588. Gels. 2025. PMID: 40868719 Free PMC article. Review.
-
A Preliminary Experimental Study of Polydimethylsiloxane (PDMS)-To-PDMS Bonding Using Oxygen Plasma Treatment Incorporating Isopropyl Alcohol.Polymers (Basel). 2023 Feb 17;15(4):1006. doi: 10.3390/polym15041006. Polymers (Basel). 2023. PMID: 36850290 Free PMC article.
-
Advances and Innovations of 3D Bioprinting Skin.Biomolecules. 2022 Dec 27;13(1):55. doi: 10.3390/biom13010055. Biomolecules. 2022. PMID: 36671440 Free PMC article. Review.
-
Improved and Highly Reproducible Synthesis of Methacrylated Hyaluronic Acid with Tailored Degrees of Substitution.ACS Omega. 2024 Jun 6;9(24):25914-25921. doi: 10.1021/acsomega.4c00372. eCollection 2024 Jun 18. ACS Omega. 2024. PMID: 38911780 Free PMC article.
References
-
- Koehler J., Brandl F.P., Goepferich A.M. Hydrogel Wound Dressings for Bioactive Treatment of Acute and Chronic Wounds. Eur. Polym. J. 2018;100:1–11. doi: 10.1016/j.eurpolymj.2017.12.046. - DOI
-
- Beldon P. Basic science of wound healing. Surgery (Oxford) 2010;28:409–412. doi: 10.1016/j.mpsur.2010.05.007. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

