The Biochemical Properties of Manganese in Plants
- PMID: 31569811
- PMCID: PMC6843630
- DOI: 10.3390/plants8100381
The Biochemical Properties of Manganese in Plants
Abstract
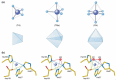
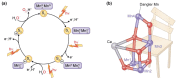
Manganese (Mn) is an essential micronutrient with many functional roles in plant metabolism. Manganese acts as an activator and co-factor of hundreds of metalloenzymes in plants. Because of its ability to readily change oxidation state in biological systems, Mn plays and important role in a broad range of enzyme-catalyzed reactions, including redox reactions, phosphorylation, decarboxylation, and hydrolysis. Manganese(II) is the prevalent oxidation state of Mn in plants and exhibits fast ligand exchange kinetics, which means that Mn can often be substituted by other metal ions, such as Mg(II), which has similar ion characteristics and requirements to the ligand environment of the metal binding sites. Knowledge of the molecular mechanisms catalyzed by Mn and regulation of Mn insertion into the active site of Mn-dependent enzymes, in the presence of other metals, is gradually evolving. This review presents an overview of the chemistry and biochemistry of Mn in plants, including an updated list of known Mn-dependent enzymes, together with enzymes where Mn has been shown to exchange with other metal ions. Furthermore, the current knowledge of the structure and functional role of the three most well characterized Mn-containing metalloenzymes in plants; the oxygen evolving complex of photosystem II, Mn superoxide dismutase, and oxalate oxidase is summarized.
Keywords: Manganese; Mn cluster; catalysis; database; enzyme; metalloenzyme; oxalate oxidase; photosystem II; superoxide dismutase.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Figures
References
-
- Saussure T.D. Recherches Chimiques sur la Végétation. Nyon; Paris, France: 1804.
-
- McHargue J.S. The effect of manganese on the growth of wheat: A source of manganese for agricultural purposes. J. Ind. Eng. Chem. 1919;11:332–335. doi: 10.1021/ie50112a022. - DOI
-
- Loew O., Sawa S. On the action of manganese compounds on plants. Bull. Coll. Agric. Tokyo. 1902;5:161–172.
-
- Pirson A. Ernährungs- und stoffwechselphysiologische Untersuchungen an Fontinalis und Chlorella. Zeitschrift für Botanik. 1937;31:193–267.
-
- Pirson A., Tichy C., Wilhelmi G. Stoffwechsel und mineralsalzernährung Einzelliger Grünalgen. Planta. 1951;40:199–253. doi: 10.1007/BF01914856. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

