Leveraging a large microbial strain collection for natural product discovery
- PMID: 31570525
- PMCID: PMC6851301
- DOI: 10.1074/jbc.REV119.006514
Leveraging a large microbial strain collection for natural product discovery
Abstract
Throughout history, natural products have significantly contributed to the discovery of novel chemistry, drug leads, and tool molecules to probe and address complex challenges in biology and medicine. Recent microbial genome sequencing efforts have uncovered many microbial biosynthetic gene clusters without an associated natural product. This means that the natural products isolated to date do not fully reflect the biosynthetic potential of microbial strains. This observation has rejuvenated the natural product community and inspired a return to microbial strain collections. Mining large microbial strain collections with the most current technologies in genome sequencing, bioinformatics, and high-throughput screening techniques presents new opportunities in natural product discovery. In this review, we report on the newly expanded microbial strain collection at The Scripps Research Institute, which represents one of the largest and most diverse strain collections in the world. Two complementary approaches, i.e. structure-centric and function-centric, are presented here to showcase how to leverage a large microbial strain collection for natural product discovery and to address challenges and harness opportunities for future efforts. Highlighted examples include the discovery of alternative producers of known natural products with superior growth characteristics and high titers, novel analogs of privileged scaffolds, novel natural products, and new activities of known and new natural products. We anticipate that this large microbial strain collection will facilitate the discovery of new natural products for many applications.
Keywords: bioinformatics; biosynthesis; drug screening; function-centric approaches; genome mining; high-throughput screening (HTS); microbial strain collection; natural product; strain prioritization; structure-centric.
© 2019 Steele et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures
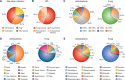

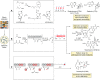

References
-
- Cimermancic P., Medema M. H., Claesen J., Kurita K., and Wieland Brown L. C., Mavrommatis K., Pati A., Godfrey P. A., Koehrsen M., Clardy J., Birren B. W., Takano E., Sali A., Linington R. G., and Fischbach M. A. (2014) Insights into secondary metabolism from a global analysis of prokaryotic biosynthetic gene clusters. Cell 158, 412–421 10.1016/j.cell.2014.06.034 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

