Tumour-intrinsic resistance to immune checkpoint blockade
- PMID: 31570880
- PMCID: PMC8499690
- DOI: 10.1038/s41577-019-0218-4
Tumour-intrinsic resistance to immune checkpoint blockade
Abstract
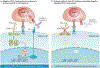
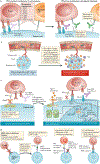
'Immune checkpoint blockade' for cancer describes the use of therapeutic antibodies that disrupt negative immune regulatory checkpoints and unleash pre-existing antitumour immune responses. Antibodies targeting the checkpoint molecules cytotoxic T lymphocyte antigen 4 (CTLA4), programmed cell death 1 (PD1) and PD1 ligand 1 (PD-L1) have had early success in the clinic, which has led to approval by the US Food and Drug Administration of multiple agents in several cancer types. Yet, clinicians still have very limited tools to discriminate a priori patients who will and will not respond to treatment. This has fuelled a wave of research into the molecular mechanisms of tumour-intrinsic resistance to immune checkpoint blockade, leading to the rediscovery of biological processes critical to antitumour immunity, namely interferon signalling and antigen presentation. Other efforts have shed light on the immunological implications of canonical cancer signalling pathways, such as WNT-β-catenin signalling, cell cycle regulatory signalling, mitogen-activated protein kinase signalling and pathways activated by loss of the tumour suppressor phosphoinositide phosphatase PTEN. Here we review each of these molecular mechanisms of resistance and explore ongoing approaches to overcome resistance to immune checkpoint blockade and expand the spectrum of patients who can benefit from immune checkpoint blockade.
Conflict of interest statement
Competing interests
A.K. has no competing financial or other conflicts of interest. A.R. has received honoraria from consulting with Amgen, Bristol-Myers Squibb, Chugai, Genentech, Merck, Novartis and Roche and is or has been a member of the scientific advisory board and holds stock in Advaxis, Arcus Biosciences, Bioncotech Therapeutics, Compugen, CytomX, Five Prime, FLX-Bio, ImaginAb, Isoplexis, Kite-Gilead, Lutris Pharma, Merus, PACT Pharma, Rgenix and Tango Therapeutics.
Figures



References
-
- Pitt JM et al. Resistance mechanisms to immune-checkpoint blockade in cancer: tumor-intrinsic and -extrinsic factors. Immunity 44, 1255–1269 (2016). - PubMed
-
- Rizvi NA et al. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 348, 124–128 (2015). - PMC - PubMed
-
Association of mutational burden and sensitivity to immune checkpoint blockade has been observed across malignancies, including lung cancer. This study provides further support for the importance of tumour-intrinsic biology for sensitivity to immune checkpoint blockade.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

