Limited Progress in Hepatorenal Syndrome (HRS) Reversal and Survival 2002-2018: A Systematic Review and Meta-Analysis
- PMID: 31571102
- PMCID: PMC7103565
- DOI: 10.1007/s10620-019-05858-2
Limited Progress in Hepatorenal Syndrome (HRS) Reversal and Survival 2002-2018: A Systematic Review and Meta-Analysis
Abstract
Introduction: Type 1 hepatorenal syndrome (HRS) is a fatal complication of cirrhosis. Treatments trend toward HRS reversal, but few show clear mortality benefit. We sought to quantify the progress-or lack thereof-in improving outcomes of type 1 HRS over time.
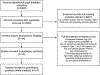
Methods: We performed a systematic review and meta-analysis for randomized controlled trials (RCTs) comparing type 1 HRS outcomes including (a) overall survival (liver transplant-free survival if reported) and (b) HRS reversal. Each study arm was analyzed separately to look at changes in outcomes over time. RCTs published comparing medical treatments for type 1 HRS were searched using several databases through July 31, 2019.
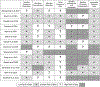
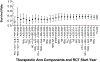
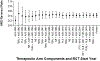
Results: Fourteen RCTs (28 arms) involving 778 participants enrolled between 2002 and 2018 were included. Twelve RCTs measured HRS reversal. In conjunction with albumin (or plasma expander), the most common medications used were terlipressin (13 arms), antibiotics (7), norepinephrine (6), dopamine (4), and midodrine/octreotide (3). Pooled survival rate was 34.6% (95% CI 26.4-43.8), and pooled HRS reversal rate was 42.8% (95% CI 34.2-51.9). Regression analyzing the incremental effect of the year the RCT was initiated showed that more recent studies were not associated with improved survival (OR 1.02, 95% CI 0.94-1.11, p = 0.66) or HRS reversal rates (OR 1.03, 95% CI 0.96-1.11, p = 0.41). There was no survival improvement when RCTs with endpoints assessed ≤ or > 1 month were analyzed separately with respective OR of 1.07 (95% CI 0.95-1.20, p = 0.26) and 0.97 (95% CI 0.85-1.12, p = 0.70).
Conclusion: Outcomes have not improved for patients with type 1 HRS since 2002. There is a need to improve prevention and treatment of type 1 HRS.
Keywords: Acute kidney injury; Albumin; Antibiotics; Cirrhosis; Hepatorenal syndrome; Hypertension; Liver cirrhosis; Portal; Renal failure; Vasopressors.
Conflict of interest statement
Figures


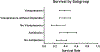

Comment in
-
Terlipressin for Type 1 Hepatorenal Syndrome.Dig Dis Sci. 2020 Aug;65(8):2454-2455. doi: 10.1007/s10620-020-06370-8. Epub 2020 Jun 8. Dig Dis Sci. 2020. PMID: 32514642 No abstract available.
References
-
- Alessandria C, Ozdogan O, Guevara M, Restuccia T, Jimenez W, Arroyo V, Rodes J, et al. MELD score and clinical type predict prognosis in hepatorenal syndrome: relevance to liver transplantation. Hepatology 2005;41:1282–1289. - PubMed
-
- Cardenas A Hepatorenal syndrome: a dreaded complication of end-stage liver disease. Am J Gastroenterol 2005;100:460–467. - PubMed
-
- A F. Clinical Report on hydro-pneumoperitoneum based on an analysis of forty-six cases. Am J Med Sci;43:306–339.
-
- Epstein M, Berk DP, Hollenberg NK, Adams DF, Chalmers TC, Abrams HL, Merrill JP. Renal failure in the patient with cirrhosis: the role of active vasoconstriction. The American journal of medicine 1970;49:175–185. - PubMed
-
- Wong Patrick Y. M GC, Spielberg Alan, Milora Robert V., Balint John A.. The Hepatorenal Syndrome. Gastroenterology 1979;77:1326–1334. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

