Serum IFI16 and anti-IFI16 antibodies in psoriatic arthritis
- PMID: 31571199
- PMCID: PMC6904656
- DOI: 10.1111/cei.13376
Serum IFI16 and anti-IFI16 antibodies in psoriatic arthritis
Abstract
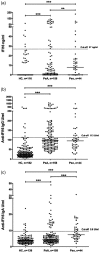
Nuclear interferon-inducible protein 16 (IFI16) and anti-IFI16 antibodies have been detected in subjects with several rheumatic diseases, often correlating with disease severity, and in this study we investigated their prevalence and clinical associations in psoriatic arthritis (PsA) compared to psoriasis (Pso). We tested sera and synovial fluids of patients with PsA for IFI16 protein levels by capture enzyme-linked immunosorbent assay (ELISA) and for anti-IFI16 immunoglobulin (Ig)G and IgA by ELISA, protein radio-immunoprecipitation and immunoprecipitation-Western blot of IgG. Sera from patients with Pso and healthy subjects were used as controls, and in a subgroup of patients with PsA we also studied sera after treatment with etanercept. IFI16 was detectable in the sera of 66% of patients with Pso, 46% with PsA and 19% of controls. Among PsA cases, 51% of IFI16-positive cases had elevated levels of C-reactive protein (CRP) compared to 31% of patients with undetectable IFI16. Anti-IFI16 of both IgG and IgA isoforms were detected with significantly higher frequency in PsA and Pso compared to healthy controls, with higher IgG titres in patients with elevated C-reactive protein (CRP) (P = 0·015). Immunoprecipitation confirmed the presence of anti-IFI16 IgG antibodies and these preferentially recognized epitopes outside the N-terminus of the protein. Lastly, IFI16 was detected in one of seven and anti-IFI16 in three of seven synovial fluids from patients with PsA. Therefore, IFI16 and anti-IFI16 are detectable in serum and synovial fluid of PsA patients, especially in cases of elevated CRP.
Keywords: IFI16; anti-IFI16 antibodies; psoriatic arthritis.
© 2019 British Society for Immunology.
Conflict of interest statement
The authors have no competing interests to declare.
Figures


Similar articles
-
Circulating Interferon-Inducible Protein IFI16 Correlates With Clinical and Serological Features in Rheumatoid Arthritis.Arthritis Care Res (Hoboken). 2016 Apr;68(4):440-5. doi: 10.1002/acr.22695. Arthritis Care Res (Hoboken). 2016. PMID: 26316393
-
Synovial fluid levels of anti-cyclic citrullinated peptide antibodies and IgA rheumatoid factor in rheumatoid arthritis, psoriatic arthritis, and osteoarthritis.Arthritis Rheum. 2006 Feb 15;55(1):53-6. doi: 10.1002/art.21691. Arthritis Rheum. 2006. PMID: 16463412
-
Anti-LL37 Antibodies Are Present in Psoriatic Arthritis (PsA) Patients: New Biomarkers in PsA.Front Immunol. 2018 Sep 12;9:1936. doi: 10.3389/fimmu.2018.01936. eCollection 2018. Front Immunol. 2018. PMID: 30279686 Free PMC article.
-
Mislocalization of the interferon inducible protein IFI16 by environmental insults: implications in autoimmunity.Cytokine Growth Factor Rev. 2015 Apr;26(2):213-9. doi: 10.1016/j.cytogfr.2014.10.003. Epub 2014 Oct 30. Cytokine Growth Factor Rev. 2015. PMID: 25466628 Review.
-
The new era for the treatment of psoriasis and psoriatic arthritis: perspectives and validated strategies.Autoimmun Rev. 2014 Jan;13(1):64-9. doi: 10.1016/j.autrev.2013.08.006. Epub 2013 Sep 8. Autoimmun Rev. 2014. PMID: 24021172 Review.
Cited by
-
Genome-wide identification of dysregulated alternative splicing and RNA-binding proteins involved in atopic dermatitis.Front Genet. 2024 Mar 1;15:1287111. doi: 10.3389/fgene.2024.1287111. eCollection 2024. Front Genet. 2024. PMID: 38495671 Free PMC article.
-
Precision Medicine in Rheumatology: The Role of Biomarkers in Diagnosis and Treatment Optimization.J Clin Med. 2025 Mar 4;14(5):1735. doi: 10.3390/jcm14051735. J Clin Med. 2025. PMID: 40095875 Free PMC article. Review.
-
Toll-like receptor 4-mediated inflammation triggered by extracellular IFI16 is enhanced by lipopolysaccharide binding.PLoS Pathog. 2020 Sep 9;16(9):e1008811. doi: 10.1371/journal.ppat.1008811. eCollection 2020 Sep. PLoS Pathog. 2020. PMID: 32903274 Free PMC article.
-
The PYRIN domain is required for TLR4-mediated inflammation by PYHIN family members.iScience. 2025 Apr 11;28(5):112413. doi: 10.1016/j.isci.2025.112413. eCollection 2025 May 16. iScience. 2025. PMID: 40454096 Free PMC article.
-
Renal interferon-inducible protein 16 expression is associated with disease activity and prognosis in lupus nephritis.Arthritis Res Ther. 2023 Jul 1;25(1):112. doi: 10.1186/s13075-023-03094-8. Arthritis Res Ther. 2023. PMID: 37393341 Free PMC article.
References
-
- Sakkas LI, Bogdanos DP. Are psoriasis and psoriatic arthritis the same disease? The IL‐23/IL‐17 axis data. Autoimmun Rev 2017; 16:10–5. - PubMed
-
- Barnas JL, Ritchlin CT. Etiology and pathogenesis of psoriatic arthritis. Rheum Dis Clin North Am 2015; 41:643–63. - PubMed
-
- Higgs BW, Liu Z, White B et al Patients with systemic lupus erythematosus, myositis, rheumatoid arthritis and scleroderma share activation of a common type I interferon pathway. Ann Rheum Dis 2011; 70:2029–36. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

