Can mouse models mimic sporadic Alzheimer's disease?
- PMID: 31571648
- PMCID: PMC6921354
- DOI: 10.4103/1673-5374.266046
Can mouse models mimic sporadic Alzheimer's disease?
Abstract
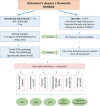
Alzheimer's disease (AD) is a progressive neurodegenerative disorder and the most common form of dementia worldwide. As age is the main risk factor, > 97% of all AD cases are of sporadic origin, potentiated by various risk factors associated with life style and starting at an age > 60 years. Only < 3% of AD cases are of genetic origin caused by mutations in the amyloid precursor protein or Presenilins 1 or 2, and symptoms already start at an age < 30 years. In order to study progression of AD, as well as therapeutic strategies, mouse models are state-of-the-art. So far many transgenic mouse models have been developed and used, with mutations in the APP or presenilin or combinations (3×Tg, 5×Tg). However, such transgenic mouse models more likely mimic the genetic form of AD and no information can be given how sporadic forms develop. Several risk genes, such as Apolipoprotein E4 and TREM-2 enhance the risk of sporadic AD, but also many risk factors associated with life style (e.g., diabetes, hypercholesterolemia, stress) may play a role. In this review we discuss the current situation regarding AD mouse models, and the problems to develop a sporadic mouse model of AD.
Keywords: Alzheimer’s disease; beta-amyloid; cerebral amyloid angiopathy; cognitive impairment; sporadic and genetic mouse models; tau; vascular risk factors.
Conflict of interest statement
None
Figures
References
-
- Balducci C, Forloni G. APP transgenic mice: their use and limitations. Neuromolecular Med. 2011;13:117–137. - PubMed
-
- Becerril-Ortega J, Bordji K, Fréret T, Rush T, Buisson A. Iron overload accelerates neuronal amyloid-β production and cognitive impairment in transgenic mice model of Alzheimer’s disease. Neurobiol Aging. 2014;35:2288–2301. - PubMed
-
- Broquères-You D, Dere E, Benessiano J, Lévy BI, Poittevin M, Mariani J, Cifuentes D, Kubis N, Merkulova-Rainon T, Bonnin P, Pocard M. Hypertension accelerates the progression of Alzheimer-like pathology in a mouse model of the disease. Hypertension. 2014;65:218–224. - PubMed
-
- Carmona S, Zahs K, Wu E, Dakin K, Bras J, Guerreiro R. The role of TREM2 in Alzheimer’s disease and other neurodegenerative disorders. Lancet Neurol. 2018;17:721–730. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

