Prevalence of cardiac comorbidities, and their underdetection and contribution to exertional symptoms in COPD: results from the COSYCONET cohort
- PMID: 31571852
- PMCID: PMC6759215
- DOI: 10.2147/COPD.S209343
Prevalence of cardiac comorbidities, and their underdetection and contribution to exertional symptoms in COPD: results from the COSYCONET cohort
Abstract
Background: A substantial prevalence of cardiovascular disease is known for COPD, but detection of its presence, relationship to functional findings and contribution to symptoms remains challenging. The present analysis focusses on the cardiovascular contribution to COPD symptoms and their relationship to the patients' diagnostic status, medication and echocardiographic findings.
Methods: Patients from the COPD cohort COSYCONET with data on lung function, including FEV1, residual volume/total lung capacity (RV/TLC) ratio, diffusing capacity TLCO, and echocardiographic data on left ventricular ejection fraction (LVEF) and end-diastolic diameter (LVEDD), medical history, medication, modified British Medical Research Council dyspnea scale (mMRC) and Saint Georges Respiratory Questionnaire (SGRQ) were analyzed.
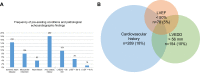
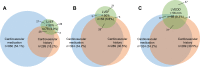
Results: A total of 1591 patients (GOLD 0-4: n=230/126/614/498/123) fulfilled the inclusion criteria. Ischemic heart disease, myocardial infarction or heart failure were reported in 289 patients (18.2%); 860 patients (54%) received at least one cardiovascular medication, with more than one in many patients. LVEF<50% or LVEDD>56 mm was found in 204 patients (12.8%), of whom 74 (36.3%) had neither a cardiovascular history nor medication. Among 948 patients (59.6%) without isolated hypertension, there were 21/55 (38.2%) patients with LVEF<50% and 47/88 (53.4%) with LVEDD>56 mm, who lacked both a cardiac diagnosis and medication. LVEDD and LVEF were linked to medical history; LVEDD was dependent on RV/TLC and LVEF on FEV1. Exertional COPD symptoms were best described by mMRC and the SGRQ activity score. Beyond lung function, an independent link from LVEDD on symptoms was revealed.
Conclusion: A remarkable proportion of patients with suspicious echocardiographic findings were undiagnosed and untreated, implying an increased risk for an unfavorable prognosis. Cardiac size and function were dependent on lung function and only partially linked to cardiovascular history. Although the contribution of LV size to COPD symptoms was small compared to lung function, it was detectable irrespective of all other influencing factors. However, only the mMRC and SGRQ activity component were found to be suitable for this purpose.
Keywords: COPD; dyspnea; echocardiography; heart failure; medication; symptoms.
© 2019 Alter et al.
Conflict of interest statement
Peter Alter, Barbara A Mayerhofer, Kathrin Kahnert, Henrik Watz, Benjamin Waschki, Frank Biertz, and Rudolf A Jörres report no conflicts of interest in this work. Stefan Andreas report grants and personal fees from Boehringer Ing and Pfizer, and personal fees from Novartis, Astra Zeneca, GSK, Chiesi, and Merini, outside the submitted work. Robert Bals report grants from German Federal Ministry of Education and Research (BMBF) Competence Network Asthma and COPD (ASCONET), during the conduct of the study, and grants and personal fees from AstraZeneca, Novartis, and Boehringer Ingelheim, and personal fees from GlaxoSmithKline, Grifols, and CSL Behring, outside the submitted work. Claus F Vogelmeier report grants and personal fees from AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Grifols, and Novartis, personal fees from CSL Behring, Chiesi, Menarini, Mundipharma, Teva, and Cipla, and grants from Bayer Schering Pharma AG, MSD, and Pfizer, outside the submitted work. The authors report no other conflicts of interest regarding this work.
Figures


References
-
- Greulich T, Weist BJD, Koczulla AR, et al. Prevalence of comorbidities in COPD patients by disease severity in a German population. Respir Med. 2017;132:132–138. - PubMed
-
- Triest FJ, Franssen FM, Spruit MA, Groenen MT, Wouters EF, Vanfleteren LE. Poor agreement between chart-based and objectively identified comorbidities of COPD. Eur Respir J. 2015;46(5):1492–1495. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

