Co-administration of aspirin and adipose-derived stem cell conditioned medium improves the functional recovery of the optic pathway in a lysolecithin-induced demyelination model
- PMID: 31571884
- PMCID: PMC6756276
- DOI: 10.2147/NDT.S218594
Co-administration of aspirin and adipose-derived stem cell conditioned medium improves the functional recovery of the optic pathway in a lysolecithin-induced demyelination model
Abstract
Introduction: Based on beneficial effects of aspirin and mesenchymal stem cells (MSCs) on myelin repair, in a preset study, effects of co-administration of aspirin and conditioned medium from adipose tissue-derived stem cells (ADSC-CM) on functional recovery of optic pathway, demyelination levels, and astrocytes' activation were evaluated in a lysolecithin (LPC)-induced demyelination model of optic chiasm.
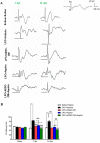
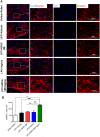
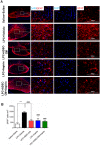
Methods: LPC (1%, 2 µL) was injected into the rat optic chiasm and animals underwent daily intraperitoneal (i.p.) injections of ADSCs-CM and oral gavage of aspirin at a dose of 25 mg/kg for 14 days post LPC injection. The conductivity of visual signals was assessed using visual evoked potential recordings (VEPs) before LPC injection and on days 7 and 14 post lesion. Immunostaining against PDGFRα as oligodendrocyte precursor cells marker, MOG as mature myelin marker, and GFAP as astrocyte marker was performed on brain sections at day 14 post LPC injection. FluoroMyelin staining was also used to measure the extent of demyelination areas.
Results: Our results showed that administration of ADSCs-CM and aspirin significantly reduced the latency of VEP waves in LPC receiving animals. In addition, demyelination levels and GFAP expressing cells were attenuated while the number of oligodendrocyte precursor cells significantly increased in rats treated with ADSCs-CM and aspirin.
Conclusion: Overall, our results suggest that co-administration of ADSCs-CM and aspirin improves the functional recovery of optic pathway through amelioration of astrocyte activation and attenuation of demyelination level.
Keywords: aspirin; conditioned medium; demyelination; lysolecithin; mesenchymal stem cells; optic chiasm.
© 2019 Galeshi et al.
Conflict of interest statement
The authors declare no conflict of interest related to this study.
Figures



References
LinkOut - more resources
Full Text Sources
Miscellaneous

