Functional characteristics of CYP3A4 allelic variants on the metabolism of loperamide in vitro
- PMID: 31571937
- PMCID: PMC6750855
- DOI: 10.2147/IDR.S215129
Functional characteristics of CYP3A4 allelic variants on the metabolism of loperamide in vitro
Abstract
Background: Cytochrome P450 3A4 (CYP3A4) appears to be genetically polymorphic, which in turn contributes to interindividual variability in response to therapeutic drugs. Loperamide, identified as a CYP3A4 substrate, is prone to misuse and abuse and has high risks of life-threatening cardiotoxicity.
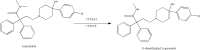
Methods: Thus, this study is designed to evaluate the enzymatic characteristics of 29 CYP3A4 alleles toward loperamide in vitro, including the 7 novel CYP3A4 variants (*28-*34). The incubation system (containing CYP3A4 enzyme, cytochrome b5, 0.5-20 μM loperamide, potassium phosphate buffer and nicotinamide adenine dinucleotide phosphate) was subject to 40-mins incubation at 37°C and the concentrations of N-demethylated loperamide were quantified by UPLC-MS/MS.
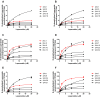
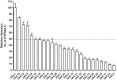
Results: As a result, CYP3A4.6, .17, .20 and .30 showed extremely low activity or no activity and the rest of CYP3A4 variants presented varying degrees of decrements in catalytical activities when compared with CYP3A4.1.
Conclusion: As the first study to identify the properties of these CYP3A4 variants toward loperamide metabolism, our investigation may establish the genotype-phenotype relationship for loperamide, predict an individual's capability in response to loperamide, and provide some guidance of clinical medication and treatment for loperamide.
Keywords: CYP3A4; cardiotoxicity; genetic polymorphism; interindividual variability; loperamide; misuse and abuse; personalized treatment.
© 2019 Lin et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures
References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

