Comparison of the changes in the factors associated with the renal prognosis of non-elderly and elderly subjects treated with empagliflozin- a retrospective observation study in Japanese patients with type 2 diabetes
- PMID: 31571954
- PMCID: PMC6750868
- DOI: 10.2147/DMSO.S221655
Comparison of the changes in the factors associated with the renal prognosis of non-elderly and elderly subjects treated with empagliflozin- a retrospective observation study in Japanese patients with type 2 diabetes
Abstract
Purpose: The factors associated with the renal prognosis over six months after the initiation of empagliflozin were compared between the non-elderly and elderly Japanese patients with type 2 diabetes.
Patients and methods: In total, 132 patients treated with empagliflozin (10 mg, once daily) were studied as the safety analysis set. One hundred ten subjects whose medications were not changed during the observation period were investigated as the full analysis set to assess the effectiveness. The subjects were divided into two groups: non-elderly subjects (n=72) of<65 years of age and elderly subjects (n=38) of≥65 years of age.
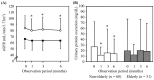
Results: Although the body weight and HbA1c, AST, ALT and γ-GTP levels were significantly reduced in both the non-elderly and elderly subjects, blood pressure, eGFR and urinary protein excretion were only significantly decreased in the non-elderly subjects. The hemoglobin, hematocrit and serum HDL-cholesterol levels were significantly elevated in both groups. The change in eGFR showed a significant positive association with the change in blood pressure. The change in urinary protein excretion tended to be correlated with the change in blood pressure.
Conclusion: Although renoprotective effects might be limited, empagliflozin can safely and effectively improve metabolic parameters, even in elderly subjects.
Keywords: elderly; empagliflozin; renal impairment; renoprotection; sodium-glucose cotransporter 2 inhibitor; type 2 diabetes mellitus.
© 2019 Ito et al.
Conflict of interest statement
H Ito has received lecture fees from Eli Lilly Japan KK, Boehringer Ingelheim, Takeda Pharmaceutical Company Ltd., Sanofi KK, Novo Nordisk Pharma Ltd., MSD KK, Novartis Pharma KK, Astellas Pharma, Daiichi Sankyo Company, Terumo Corporation, Mochida Pharmaceuticals, Teijin Pharma, Kissei Pharmaceuticals, Kowa Pharmaceuticals, Mitsubishi Tanabe Pharma Corporation, Sanwa Kagaku Kenkyusho, Dainippon Sumitomo Pharma, AstraZeneca KK, Kyowa Hakko Kirin, Shionogi and Co, Taisho Toyama Pharmaceutical Co., Ltd., Otsuka Pharmaceutical Co., Ltd., Bayer Yakuhin, Ltd., and Santen Pharmaceutical Co., Ltd., and has received consulting fee from Becton, Dickinson and Company. S Matsumoto has received lecture fees from Novo Nordisk Pharma Ltd., Astellas Pharma, and AstraZeneca KK. S Nishio has received lecture fees from Sanofi KK, Taisho Toyama Pharmaceutical Co., Ltd., Kyowa Hakko Kirin, Bayer Yakuhin, Ltd., and Mitsubishi Tanabe Pharma Corporation. S Antoku has received lecture fees from Kyowa Hakko Kirin, Sanofi KK, Kyowa Hakko Kirin, Taisho Toyama Pharmaceutical Co., Ltd., Daiichi Sankyo Company, and Otsuka Pharmaceutical Co., Ltd. S Ando has received lecture fees from Takeda Pharmaceutical Company Ltd. T Izutsu, E Kusano, T Yamasaki, T Mori, M Togane and E Tsugami have no conflicts of interest in this work.
Figures


Similar articles
-
Pharmacokinetics, pharmacodynamics and safety of empagliflozin, a sodium glucose cotransporter 2 (SGLT2) inhibitor, in subjects with renal impairment.Diabetes Obes Metab. 2014 Mar;16(3):215-22. doi: 10.1111/dom.12182. Epub 2013 Aug 19. Diabetes Obes Metab. 2014. PMID: 23859488 Clinical Trial.
-
Renoprotective Effects of Additional SGLT2 inhibitor Therapy in Patients With Type 2 Diabetes Mellitus and Chronic Kidney Disease Stages 3b-4: A Real World Report From A Japanese Specialized Diabetes Care Center.J Clin Med Res. 2019 Apr;11(4):267-274. doi: 10.14740/jocmr3761. Epub 2019 Mar 18. J Clin Med Res. 2019. PMID: 30937117 Free PMC article.
-
The kidney and cardiovascular outcome trials.J Diabetes. 2018 Feb;10(2):88-89. doi: 10.1111/1753-0407.12616. J Diabetes. 2018. PMID: 29031006
-
Empagliflozin: a sodium-glucose cotransporter 2 inhibitor for treatment of type 2 diabetes.Am J Health Syst Pharm. 2015 Nov 15;72(22):1943-54. doi: 10.2146/ajhp150071. Am J Health Syst Pharm. 2015. PMID: 26541949 Review.
-
Empagliflozin: a new sodium-glucose co-transporter 2 (SGLT2) inhibitor for the treatment of type 2 diabetes.Drugs Context. 2014 Jun 11;3:212262. doi: 10.7573/dic.212262. eCollection 2014. Drugs Context. 2014. PMID: 24991224 Free PMC article. Review.
Cited by
-
Different renoprotective effects of luseogliflozin depend on the renal function at the baseline in patients with type 2 diabetes: A retrospective study during 12 months before and after initiation.PLoS One. 2021 Mar 15;16(3):e0248577. doi: 10.1371/journal.pone.0248577. eCollection 2021. PLoS One. 2021. PMID: 33720983 Free PMC article. Clinical Trial.
-
Phase Angle Evaluated by a Bioimpedance Analysis Is Closely Related to Diabetic Nephropathy and Peripheral Neuropathy in Patients with Type 2 Diabetes.JMA J. 2025 Jul 15;8(3):925-935. doi: 10.31662/jmaj.2025-0071. Epub 2025 Jun 27. JMA J. 2025. PMID: 40786473 Free PMC article.
-
Renal outcomes with sodium-glucose cotransporters 2 inhibitors.Front Endocrinol (Lausanne). 2022 Dec 1;13:1063341. doi: 10.3389/fendo.2022.1063341. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 36531469 Free PMC article. Review.
-
Changes in the estimated glomerular filtration rate and predictors of the renal prognosis in Japanese patients with type 2 diabetes: A retrospective study during the 12 months after the initiation of tofogliflozin.PLoS One. 2023 Sep 21;18(9):e0292014. doi: 10.1371/journal.pone.0292014. eCollection 2023. PLoS One. 2023. PMID: 37733761 Free PMC article.
-
Anemia combined with albuminuria increases the risk of cardiovascular and renal events, regardless of a reduced glomerular filtration rate, in patients with type 2 diabetes: a prospective observational study.Diabetol Int. 2023 May 30;14(4):344-355. doi: 10.1007/s13340-023-00637-x. eCollection 2023 Oct. Diabetol Int. 2023. PMID: 37781474 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

