Gemcitabine, cisplatin, prednisone, and thalidomide for relapse and refractory peripheral T-cell lymphoma: a retrospective study from China
- PMID: 31571985
- PMCID: PMC6749983
- DOI: 10.2147/CMAR.S215585
Gemcitabine, cisplatin, prednisone, and thalidomide for relapse and refractory peripheral T-cell lymphoma: a retrospective study from China
Abstract
Background: Peripheral T-cell lymphoma (PTCL) is often prone to relapse and progression even after formal first-line treatment, and there is no standard regimen for second-line treatment. What is more, the activity of thalidomide against this type of lymphoma is unknown.
Purpose: The objective of this study was to evaluate the efficacy and safety of GDPT regimen in the treatment of relapsed/refractory peripheral T-cell lymphoma.
Patients and methods: In this retrospective study, gemcitabine, cisplatin, prednisone, and thalidomide (GDPT) combination regimen was used as salvage protocol for PTCL that failed in first-line treatment for 29 patients and it was scheduled to give 6 cycles of GDPT therapy in order to better evaluate the efficacy unless there was evidence of disease progression, unacceptable toxicities, or refusal by the patient.
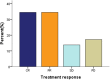
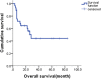
Results: After a total of 106 cycles of GDPT regimen were administered, the result showed that the disease control rate (DCR) achieved 82.8% and overall response rate (ORR) reached 69.0% with 34.5% complete remission (CR) and 34.5% partial remission (PR). The median progression-free survival (PFS) was 10.0 months (95% CI 6.6-13.4) and median OS was 28.0 months (95% CI 19.2-36.8). And the 1-year PFS rate and 1-year OS rate were 43.6% and 64.6%, respectively. Both hematologic and non-hematologic toxicities were moderate and well tolerated. There was no treatment-related death.
Conclusion: Thalidomide in combination with gemcitabine, cisplatin, prednisone regimen is a new promising approach to treating patients with relapse and refractory PTCL.
Keywords: gemcitabine; peripheral T-cell lymphoma; salvage chemotherapy; thalidomide.
© 2019 Liu et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures

References
LinkOut - more resources
Full Text Sources
Research Materials

