Sex, Age, and Handedness Modulate the Neural Correlates of Active Learning
- PMID: 31572114
- PMCID: PMC6749092
- DOI: 10.3389/fnins.2019.00961
Sex, Age, and Handedness Modulate the Neural Correlates of Active Learning
Abstract
Background: Self-generation of material compared to passive learning results in mproved memory performance; this may be related to recruitment of a fronto-temporal encoding network. Using a verbal paired-associate learning fMRI task, we examined the effects of sex, age, and handedness on the neural correlates of self-generation.
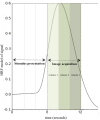
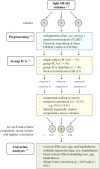
Methods: Data from 174 healthy English-speaking participants (78M, 56 atypically handed; ages 19-76) were preprocessed using AFNI and FSL. Independent component analysis was conducted using GIFT (Group ICA fMRI Toolbox). Forty-one independent components were temporally sorted by task time series. Retaining correlations (r > 0.25) resulted in three task-positive ("generate") and three task-negative ("read") components. Using participants' back-projected components, we evaluated the effects of sex, handedness, and aging on activation lateralization and localization in task-relevant networks with two-sample t-tests. Further, we examined the linear relationship between sex and neuroimaging data with multiple regression, covarying for scanner, age, and handedness.
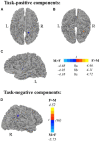
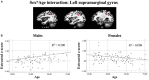
Results: Task-positive components identified using ICA revealed a fronto-parietal network involved with self-generation, while task-negative components reflecting passive reading showed temporo-occipital involvement. Compared to older adults, younger adults exhibited greater task-positive involvement of the left inferior frontal gyrus and insula, whereas older adults exhibited reduced prefrontal lateralization. Greater involvement of the left angular gyrus in task-positive encoding networks among right-handed individuals suggests the reliance on left dominant semantic processing areas may be modulated by handedness. Sex effects on task-related encoding networks while controlling for age and handedness suggest increased right hemisphere recruitment among males compared to females, specifically in the paracentral lobe during self-generation and the suparmarginal gyrus during passive reading.
Implications: Identified neuroimaging differences suggest that sex, age, and handedness are factors in the differential recruitment of encoding network regions for both passive and active learning.
Keywords: age; associate learning; fMRI; handedness; sex; verbal memory.
Copyright © 2019 Nair, Nenert, Allendorfer, Goodman, Vannest, Mirman and Szaflarski.
Figures





Similar articles
-
Age related-changes in the neural basis of self-generation in verbal paired associate learning.Neuroimage Clin. 2015 Feb 20;7:537-46. doi: 10.1016/j.nicl.2015.02.006. eCollection 2015. Neuroimage Clin. 2015. PMID: 25844310 Free PMC article.
-
Cortical correlates of self-generation in verbal paired associate learning.Brain Res. 2012 Feb 9;1437:104-14. doi: 10.1016/j.brainres.2011.12.020. Epub 2011 Dec 16. Brain Res. 2012. PMID: 22227457 Free PMC article. Clinical Trial.
-
Handedness-dependent functional organizational patterns within the bilateral vestibular cortical network revealed by fMRI connectivity based parcellation.Neuroimage. 2018 Sep;178:224-237. doi: 10.1016/j.neuroimage.2018.05.018. Epub 2018 May 19. Neuroimage. 2018. PMID: 29787866
-
Activation of brain regions using task-state FMRI in patients with mild traumatic brain injury: a meta-analysis.Int J Clin Exp Pathol. 2020 Dec 1;13(12):2918-2926. eCollection 2020. Int J Clin Exp Pathol. 2020. PMID: 33425093 Free PMC article. Review.
-
Neural Correlates of Verbal Working Memory: An fMRI Meta-Analysis.Front Hum Neurosci. 2019 Jun 12;13:180. doi: 10.3389/fnhum.2019.00180. eCollection 2019. Front Hum Neurosci. 2019. PMID: 31244625 Free PMC article.
Cited by
-
Interrogation of the human cortical peptidome uncovers cell-type specific signatures of cognitive resilience against Alzheimer's disease.Sci Rep. 2024 Mar 26;14(1):7161. doi: 10.1038/s41598-024-57104-z. Sci Rep. 2024. PMID: 38531951 Free PMC article.
-
White Matter Language Pathways and Language Performance in Healthy Adults Across Ages.Front Neurosci. 2019 Nov 1;13:1185. doi: 10.3389/fnins.2019.01185. eCollection 2019. Front Neurosci. 2019. PMID: 31736704 Free PMC article.
-
Brain Functional Alternations of the Pain-related Emotional and Cognitive Regions in Patients with Chronic Shoulder Pain.J Pain Res. 2020 Mar 20;13:575-583. doi: 10.2147/JPR.S220370. eCollection 2020. J Pain Res. 2020. PMID: 32256105 Free PMC article.
-
Effects of different exercise intensities of race-walking on brain functional connectivity as assessed by functional near-infrared spectroscopy.Front Hum Neurosci. 2022 Oct 14;16:1002793. doi: 10.3389/fnhum.2022.1002793. eCollection 2022. Front Hum Neurosci. 2022. PMID: 36310841 Free PMC article.
-
The effect of the apolipoprotein E ε4 allele and olfactory function on odor identification networks.Brain Behav. 2024 May;14(5):e3524. doi: 10.1002/brb3.3524. Brain Behav. 2024. PMID: 38702902 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

